REGISTRO DOI: 10.69849/revistaft/cs10202506292205
Misael das Virgens Santana1; Francisco Edio Neves da Silva2; Wallisson Bruno de Morais Pacheco3; Joashllenny Alves de Oliveira4; Marcela Pereira Gualter5; Sérgio Henrique Costa Júnior6; Luiz Augusto de Oliveira7; José Adalmir Torres de Souza8
Abstract: The history of the Northeastern Horse, like that of other equine breeds, is closely linked to the colonization of Brazil. This species exhibits recurrent estrus in regions near the equator. Therefore, ultrasonographic evaluation of the ovaries allows for the determination of the number, location, size, and shape of ovarian follicles. In this context, the objective of this study was to evaluate the dynamics of follicular growth in Northeastern Horse mares following administration of a drug with luteolytic action. The research was conducted at Fazenda Faveira, in the municipality of Elesbão Veloso, Piauí State, using fifteen animals over a period of 22 consecutive days. The animals received two intramuscular doses (6.71 mg each) of dinoprost tromethamine, 12 days apart, for follicular growth synchronization. Forty-eight hours after the second dose, they were examined daily using a Mindray® Z6 VET ultrasound. Follicular growth monitoring considered a maximum of three follicles per ovary. Statistical analysis was performed using IBM SPSS Statistics software, version 26.0. Comparison of follicular diameters in animals that ovulated revealed no significant differences. Of the 15 animals, 66.66% exhibited follicular growth and ovulation, with an average daily follicular growth rate of 2.12 millimeters (mm). However, 33.33% did not show sufficient follicular development to reach ovulation. Northeastern Horse mares had an average follicular diameter of 37.11 mm one day before ovulation. In conclusion, the follicular growth dynamics observed in Northeastern Horse mares in this study are similar to those reported for mares of other breeds.
Keywords: equine, follicles, ultrasonography.
Introduction
The history of the Northeastern Horse, in terms of its formation as a breed, dates back to the colonization of Brazil. Like other Brazilian breeds, its origin lies in horses brought from Europe, which arrived with various colonizers during the numerous expeditions that reached Brazilian territory (PIRES et al., 2014).
Regarding reproduction, the species exhibits recurrent estrus in regions where the photoperiod does not interfere, with an average estrous cycle duration of 21 to 22 days. Equine females tend to present one or two follicular waves during the estrous cycle, with a single wave being more common. Preovulatory follicles grow approximately three to five millimeters in diameter per day (CLAES et al., 2017; GINTHER, 2017; DRIANCOURT, 2001; BRINSKO et al., 2011; FERREIRA; WISCHRAL, 2020).
Ultrasonographic evaluation of the mare’s ovaries allows for accurate determination of the number, location, size, and shape of the follicles. Approximately 24 hours before ovulation, most follicles tend to change from a spherical to a conical or pear-shaped form, and the follicular wall may appear thickened (BRINSKO et al., 2011; GINTHER et al., 2018). Based on these characterizations, the objective of this study was to evaluate the dynamics of follicular growth following the administration of a luteolysis-inducing agent in Northeastern Horse mares.
Materials and Methods
The practical activities were carried out at Fazenda Faveira, located at kilometer 138 of BR-316, situated at 06°12’07’’S and 42°08’25’’W, in the municipality of Elesbão Veloso, State of Piauí, Brazil. The climate is classified as hot semi-arid tropical, with temperatures ranging from 25°C to 36°C and rainfall ranging from 125 mm (monthly average) to 1171.5 mm (annual average). The predominant vegetation consists of arboreal and shrubby Caatinga, interspersed with patches of cerrado grassland. During the month of June, there are approximately 14 minutes more darkness compared to other months, whereas in December, daylight duration increases by approximately 31 minutes (FUNDAÇÃO CEPRO, 2001; WEATHER SPARK, n.d.).
The animals were evaluated through clinical gynecological examination, and fifteen mares were selected for the study. The experiment lasted for twenty-two consecutive days, between February and March of 2021. During this period, the animals were kept on pasture with Andropogon gayanus grass and had free access to water. The body condition score (BCS) ranged from four to six (HENNEKE et al., 1983), with a mean age of 5.4 years (±2.9) and an average body weight of 243.8 kg (±53.98).
All animals received two intramuscular doses of 6.71 mg of dinoprost tromethamine (Lutalyse®) for synchronization purposes (KAPS et al., 2021), with the first administration on Day 0 (D0) and the second on Day 12 (D12). Forty-eight hours after the second administration of dinoprost tromethamine, all mares were examined every 24 hours for 22 consecutive days using an ultrasound device (Mindray® Z6VET) equipped with a multi-frequency linear transrectal transducer calibrated at 5 MHz. The examinations aimed to characterize uterine edema on a scale from 0 to 3 (MARTÍNEZ-BOVÍ; PLAZA-DÁVILA; CUERVO-ARANGO, 2023), assess the uterine horn diameter (transverse section of the medial part of both horns), monitor follicular development, and identify the presence of a corpus luteum (CL).
Follicular growth monitoring was conducted considering a maximum of three follicles per ovary (additional follicles were quantified but not included for measurement purposes). Only follicles with a diameter greater than five millimeters were considered for measurement. Diameters were determined based on the largest antral area (anechoic region) of the follicle and calculated as the mean of the two largest dimensions (height and width), using frozen ultrasound images and the electronic measurement tool available on the device. The position, shape, and size of the follicles in each ovary were recorded on specific data sheets for subsequent retrospective analysis of the follicular growth pattern (CUERVO-ARANGO; NEWCOMBE, 2008; DELCHIARO et al., 2024).
Statistical analysis was performed using IBM SPSS Statistics software, version 26.0. Data were tested for normal distribution using the Kolmogorov-Smirnov test. Descriptive statistics were used to characterize the follicular dynamics. The mean was used as the measure of central tendency, and the standard deviation was calculated to assess the variability of the results.
Results and Discussion
After conducting the normality test, it was found that all data followed a normal distribution. On day 0 (D0), 80% of the animals had a corpus luteum, and on day 12 (D12), 46.66%. Of the 15 animals, during the study, 66.66% showed follicular growth and ovulation, with an average daily follicular growth rate of 2.12 mm. However, 33.33% did not exhibit sufficient follicular development to reach ovulation.
The luteal phase is relatively constant, lasting fourteen to fifteen days, while the estrus or follicular phase is variable, ranging from two to twelve days (BRINSKO et al., 2011). It was observed that three animals did not present a corpus luteum nor follicles with progressive growth, indicating that the animals were in anestrus, possibly of nutritional origin.
The endocrine control of the estrous cycle is regulated by the hypothalamic-pituitary gonadal axis (ALVES et al., 2019). Equines are seasonally polyestrous animals, with light being a positively influencing environmental factor. In some regions of lower latitude, mares remain cyclic throughout the entire year (FARIAS et al., 2016; OBERHAUS et al., 2018). Thus, for the animals that did not ovulate during the experimental period, it is understood that the cause is not related to photoperiod, but rather to another factor, such as a low body condition score (BCS) or a deficiency in nutrient intake or absorption.
Of the five animals that did not ovulate, one had a hemorrhagic anovulatory follicle (HAF), another presented a corpus luteum throughout the entire study period, and three showed neither a corpus luteum nor progressive follicular growth. Among the ten animals that ovulated, six exhibited follicular growth with clearly defined phases of emergence, divergence, dominance, and pre-ovulatory development. In four animals, it was not possible to assess the follicular emergence phase.
Regarding the animal that presented a hemorrhagic anovulatory follicle (HAF), Cuervo Arango; Newcombe (2012) stated that this event may occur spontaneously or be induced through systemic administration of prostaglandin synthesis inhibitors. Ultrasonographic images reveal the outcome of a cascade of clinical events that resulted in ovulatory failure. These events include intrafollicular hemorrhage, increased follicular diameter, and luteinization of the follicular wall without rupture, despite secondary ovulatory changes such as the LH surge, increased progesterone levels, or disappearance of endometrial edema.
The follicular wave phases, the day on which each phase occurred, the number of follicles, follicular diameters, and growth rates are presented in Table 1. In this table, follicular diameter was calculated based on the average of follicles from animals that ovulated and exhibited these phases during the study – representing 60% of the total. The table also includes the day each phase occurred and the average follicular growth rate during each respective phase.
Table 1. Mean follicular diameter in Nordestino mares in the state of Piauí, Brazil, during the emergence, divergence, dominance, and pre-ovulatory phases
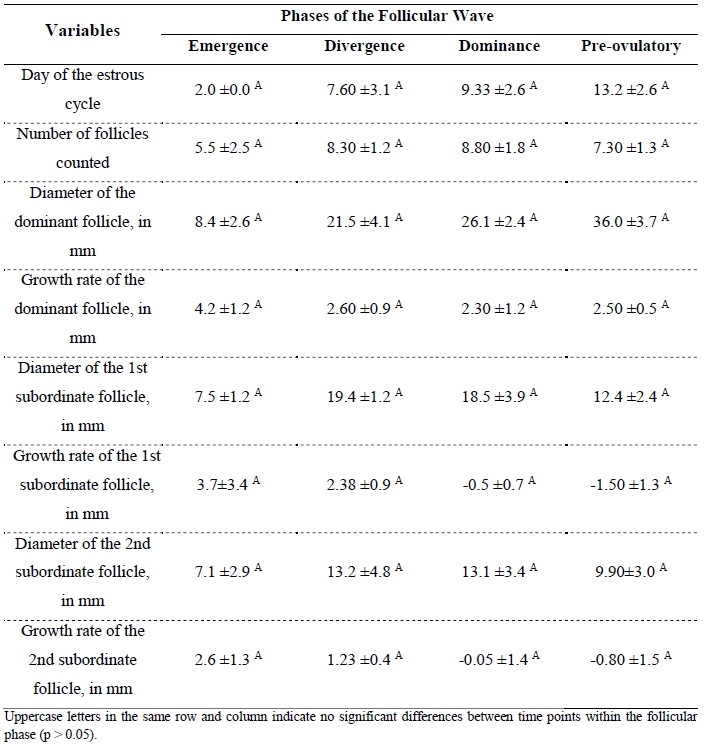
Regarding follicular diameter, it is widely used in practice as a tool to guide ovulation prediction in equines (CAVINDER et al., 2009; TAZAWA et al., 2017). The follicular growth rate observed in the animals of this study is similar to the results reported by Ramírez et al. (2010), who found a daily follicular growth of 2.04 mm.
Follicular dominance is a necessary and fundamental process to ensure a single ovulation in the equine species (GASTAL et al., 2004; GURGEL et al., 2008; GINTHER, 2017; FERREIRA et al., 2020; COELHO et al., 2023). Considering how the emergence of a dominant follicle occurs, the study of these follicular phases in Nordestino horses showed no variation in follicular emergence (Table 1), as previously described and defined by the aforementioned authors.
Ferreira et al. (2020) demonstrated that two follicles grow similarly until the larger follicle reaches 22 to 25 mm, approximately six days after the emergence of the future dominant follicle. The results obtained from the daily evaluation of follicular growth in Nordestino mares followed similar findings, as evidenced in Table 1.
The dominant follicle emerges among other follicles, exhibiting a more pronounced diameter compared to the rest, reaching dominance while the others undergo atresia. Follicular diameter and growth rate were similar to those observed in other equine breeds, such as Paso Peruano horses (RAMÍREZ et al., 2010) and Campolina horses (ZÚCCARI et al., 2013).
Table 2 displays: (i) mean diameter of the left horn (MDLH) at 28.61 ± 1.73 mm; (ii) mean diameter of the right horn (MDRH) at 28.47 ± 1.42 mm; (iii) mean total number of follicles (MTNF), showing 8 ± 2 follicles per animal; (iv) uterine edema one day prior to ovulation (EDUPO) at 1.2 ± 0.42; (v) follicular diameter one day prior to ovulation (FDPO) at 37.11 ± 5.68 mm; and (vi) mean day of ovulation (DO) at 14.9 ± 5.34 days for all animals to ovulate.
Table 2. Mean uterine horn diameters, follicle count, uterine edema, follicular diameter, and day of ovulation in Nordestino mares in the state of Piauí, Brazil
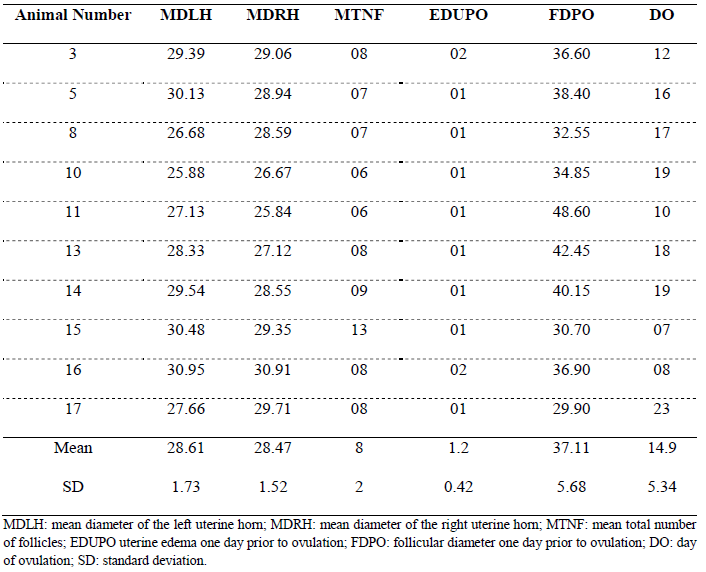
Measurements of the uterine horns are variables that are not usually recorded; however, given the limited information available on this breed in animal reproduction, they may represent an interesting parameter for future studies. Initially, observations of the uterine horn measurements showed a slight variation in the mean diameter between the right and left horns in nearly all animals. However, this asymmetry in horn diameter is not inherently related to the animals themselves, but rather to factors such as the measurement site on the uterine horn and restless animals, which complicate accurate measurement.
Although follicles were collected and not just counted as in the present study, Haag et al. (2013), in a pioneering research, demonstrated that the average number of aspirated follicles was higher in younger mares (5 to 7 years), around 27, compared to 16 follicles in older mares (14 to 21 years). Gastal et al. (2020) reported no differences between older and younger mare groups in the viability and morphological rates of primordial follicles. In light of this, the mean total follicle count of eight follicles per animal shown in Table 2 appears to be lower than the follicle numbers reported in other studies.
Regarding uterine edema, a characteristic used to assist in estimating the optimal timing for artificial insemination or controlled mating (PASCH et al., 2024), the follicular diameter averages presented in Tables 1 and 2 support this assertion. This is because when follicles reach pre-ovulatory diameter and uterine edema decreases, the animals ovulate without the need for ovulation induction with medications (TIRPAN et al., 2024).
Conclusion
The results found (follicular growth rate and pre-ovulatory follicle diameter) demonstrates the dynamics of follicular growth in Nordestino Horses are similar to those observed in studies with mares of other breeds.
Acknowledgments
The authors thank the Federal University of Piauí (UFPI), the Center for Agricultural Sciences, the Graduate Program in Animal Science, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (CAPES), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting the scholarship during the first author’s master’s degree period.
Declaration of Conflicts of Interest
The authors declare that there is no conflict of interest. The sponsors had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author Contributions
All authors contributed equally to the conception and writing of the manuscript. All authors critically reviewed the manuscript and approved the final version.
Ethics and Biosafety Committee Approval
We certify that the project entitled “Follicular Growth Dynamics and Fixed-Time Artificial Insemination (FTAI) in Nordestino Mares,” registered under number 644/2020 and under the responsibility of Prof. Dr. José Adalmir Torres de Souza from the Department of Veterinary Clinic and Surgery/CCA/UFPI, involving the production, maintenance, or use of animals belonging to the phylum Chordata, subphylum Vertebrata (excluding humans), for scientific research purposes, is in accordance with the provisions of Law No. 11,794, dated october 8, 2008, Decree No. 6,899, dated july 15, 2009, and the regulations issued by the National Council for the Control of Animal Experimentation (CONCEA). This project was approved by the Ethics Committee on Animal Use (CEUA/UFPI) of the Federal University of Piauí during the meeting held on october 2, 2020.
References
ALVES, R.G.N. et al. Influence of the cyclicity on the pregnancy rate in animals submitted to the IATF program. Brazilian Journal of Development, v.5, n.11, p.24701-24706, 2019. https://doi.org/10.34117/bjdv5n11-147.
BRINSKO, S.P. et al. Reproductive physiology of the nonpregnant mare. In: S.P. BRINSKO; T.E. BLANCHARD; D.D. VARNER; J. SCHUMACHER (Eds.), Manual of equine reproduction. 3ed, Mosby: ELSEVIER, 2011. p.16-24. https://doi.org/10.1016/C2009-0- 59988-6.
CAVINDER, C.A. et al. Variances in reproductive efficiency of mares in fat and moderate body conditions following parturition. Animal Science, v.25, n.3, p.250-255, 2009. http://dx.doi.org/10.15232/S1080-7446(15)30714-2.
CLAES, A. et al. The influence of age, antral follicle count and diestrous ovulations on estrous cycle characteristics of mares. Theriogenology, v.97, p.34-40, 2017. https://doi.org/10.1016/j.theriogenology.2017.04.019.
COELHO, L.A. et al. Seasonal variation of melatonin concentration and mRNA expression of melatonin-related genes in developing ovarian follicles of mares kept under natural photoperiods in the Southern Hemisphere. Animals, v.13, n.6, e.1063, 2023. https://doi.org/10.3390/ani13061063.
CUERVO-ARANGO, J.; NEWCOMBE, J.R. Ultrasound characteristics of experimentally induced luteinized unruptured follicles (LUF) and naturally occurring hemorrhagic anovulatory follicles (HAF) in the mare. Theriogenology, v.77, n.3, p.514-524, 2012. https://doi.org/10.1016/j.theriogenology.2011.08.026.
CUERVO-ARANGO, J.; NEWCOMBE, J.R. Repeatability of preovulatory follicular diameter and uterine edema pattern in two consecutive cycles in the mare and how they are influenced by ovulation inductors. Theriogenology, v.69, n6, p.681-687, 2008. https://doi.org/10.1016/j.theriogenology.2007.11.019
DELCHIARO, S.B. et al. Relationships between antral follicle count and reproductive characteristics of embryo-recipient mares. Journal of Equine Veterinary Science, v.134, e105029, 2024. https://doi.org/10.1016/j.jevs.2024.105029.
DRIANCOURT, M.A. Regulation of ovarian follicular dynamics in farm animals: Implications for manipulation of reproduction. Theriogenology, v.55, p.1211-1239, 2001. https://doi.org/10.1016/s0093-691x(01)00479-4.
FARIAS, L.D. et al. Indução da ovulação em éguas: Uma revisão. Revista Brasileira de Reprodução Animal, v.40, n.1, 17-21, 2016. http://cbra.org.br/pages/publicacoes/rbra/v40/n1/p17-21%20(RB611).pdf.
FERREIRA, L.E.P.A. et al. Influência da somatotropina recombinante bovina no desenvolvimento folicular e na coleta de embriões em éguas. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v.72, n.3, p.879-888, 2020. https://doi.org/10.1590/1678-4162- 10604.
FUNDAÇÃO CEPRO. (2001). Anuário estatístico do Piauí. p.1-6 http://www.cepro.pi.gov.br/download/201102/CEPRO25_b744851c89.pdf
GASTAL, E.L. et al. Harvesting, processing, and evaluation of in vitro-manipulated equine preantral follicles: A review. Theriogenology, v.156, p.283-295, 2020. https://doi.org/10.1016/j.theriogenology.2020.06.044.
GASTAL, E.L. et al. Interrelationships among follicles during the common growth phase of a follicular wave and capacity of individual follicles for dominance in mares. Reproduction, v.128, p.417-422, 2004. https://doi.org/10.1530/rep.1.00259.
GINTHER, O.J. Selection of the dominant follicle in cattle and horses. Animal Reproduction Science, v60, p.61-79, 2020. https://doi.org/10.1016/S0378-4320(00)00083-X.
GINTHER, O.J. Systemic and intrafollicular components of follicle selection in mares. Domestic Animal Endocrinology, v.59, p.116-133, 2017. https://doi.org/10.1016/j.domaniend.2016.12.005.
GINTHER, O.J. et al. Concentrations of follicle stimulating hormone associated with follicle selection, number of follicles, and ipsilateral vs contralateral relationships in mares. Theriogenology, v.113, p.159-165, 2018. https://doi.org/10.1016/j.theriogenology.2018.02.017.
GURGEL, J.R.C. et al. Dinâmica folicular em éguas: Aspectos intrafoliculares. Revista Brasileira de Reprodução Animal, v.32, n.2, p.122-132, 2008. http://www.cbra.org.br/pages/publicacoes/rbra/download/RB159%20Gurgel%20vr2%20pag1 22-132.pdf.
HAAG, K.T. et al. Quantification, morphology, and viability of equine preantral follicles obtained via the biopsy pick-up method. Theriogenology, v.79, p.599-609, 2013. doi.org/10.1016/j.theriogenology.2012.11.012.
HENNEKE, D.R. et al. Relationship between condition score, physical measurements e body fat percentage in mares. Equine Journal Veterinary, v.15, p.371-372, 1983. https://doi.org/10.1111/j.2042-3306.1983.tb01826.x.
KAPS, M. et al. Transient suppression of ovulatory ovarian function in pony mares after treatment with slow-release deslorelin implants. Domestic Animal Endocrinology, v.74, p.1- 7, 2021. https://doi.org/10.1016/j.domaniend.2020.106505.
MARTÍNEZ-BOVÍ, R.; PLAZA-DÁVILA, M.; CUERVO-ARANGO, J. The effect of dexamethasone and flunixin-meglumine on ovulation, endometrial oedema, and inter-ovulatory interval length in the mare. Theriogenology, v.197, p.57-61, 2023. https://doi.org/10.1016/j.theriogenology.2022.11.042.
OBERHAUS, E.L. et al. Effects of combined estradiol-sulpiride treatment and follicle ablation on vernal transition in mares: Evaluation of plasma and follicular fluid hormones and luteinizing hormone receptor gene expression. Journal of Equine Veterinary Science, v.64, p.69-76, 2018. https://doi.org/10.1016/j.jevs.2018.02.020.
PASCH, L. et al. Factors affecting pregnancy rates in mares bred with cryopreserved semen. Journal of Equine Veterinary Science, v.141, e105167, 2024. https://doi.org/10.1016/j.jevs.2024.105167.
PIRES, D.A.F. et al. Genetic diversity and population structure in remnant subpopulations of Nordestino Horse breed. Archivos de Zootecnia, v.63, n.242, 349-358, 2014. https://dx.doi.org/10.4321/S0004-05922014000200013.
RAMÍREZ, G.; GUTIÉRREZ, C.; RAMOS, M. Dinámica folicular en yeguas paso fino colombiano medido por ultrasonografía en la Sabana de Bogotá. Revista de Medicina Veterinaria, n.19, p.21-35, 2010. http://www.scielo.org.co/pdf/rmv/n19/n19a03.pdf.
TAZAWA, S.P. et al. Preovulatory follicle dynamics, and ovulatory and endometrial responses to different doses of hCG and prediction of ovulation in mares. Journal of Equine Veterinary Science, v.56, p.40-51, 2017. https://doi.org/10.1016/j.jevs.2017.04.008.
TIRPAN, M. et al. Pregnancy rates according to follicle diameter and uterus edema in different age groups in two consecutive ovulations in Arabian mares. Veterinarski Arhiv, v.94, p.173- 182, 2024. https://doi.org/10.24099/vet.arhiv.2471.
WEATHER SPARK – The Weather of Anywhere on Earth All Year Round. (nd). Pt.weatherspark.com. https://en.weatherspark.com/
ZÚCCARI, C.E.S.N., et al. Eficiência reprodutiva e dinâmica folicular de éguas campolina de acordo com a condição corporal. Ciência Animal Brasileira, v.14, n4, p.406-412, 2013. https://doi.org/10.5216/cab.v14i4.17693.
1Doctoral student in Tropical Animal Science – UFPI, Teresina, Piauí, Brazil; ORCID: 0000-0002-4533-1124; E-mail: misaelsantana2100@gmail.com.
2Doctoral student in Animal Science – UEMA, São Luís, Maranhão, Brazil; ORCID: 0000-0002-1517-7381.
3Resident in Large Animal Clinic and Surgery – HVU/UFPI, Teresina, Piauí, Brazil; ORCID: 0000-0002-5482-3446.
4Ph.D. in Animal Science – UFV, Viçosa, Minas Gerais, Brazil; ORCID: 0000-0001-7249-5047.
5Master’s student in Tropical Animal Science – UFPI, Teresina, Piauí, Brazil; ORCID: 0009-0004-6234-4316.
6Assistant Professor – UFPI, Teresina, Piauí, Brazil; ORCID: 0000-0002-1459-4079.
7Associate Professor – UFPI, Teresina, Piauí, Brazil; ORCID: 0000-0003-0380-8753.
8Full Professor – UFPI, Teresina, Piauí, Brazil; ORCID: 0000-0003-1346-1706.
