TRANSFORMANT LE DIAGNOSTIC ET LE TRAITEMENT DU CANCER : LE RÔLE DE L’INTELLIGENCE ARTIFICIELLE À L’ÈRE DE LA MÉDECINE DE PRÉCISION
REGISTRO DOI: 10.5281/zenodo.11118170
Ezequiel Almeida Barros1, Italo Hugo Almeida Antero2, Lucas Bragagnolo Lima3, Gabriel Pereira da Silva4, Evelly Sind Sampaio dos Santos5, Eva Carlla de Sousa Lima6, Eduardo Araujo Santana7, Pablinny da Silva Santos8, Geovania Alencar de Sousa9, Daniel Ferreira dos Santos10
ABSTRACT
INTRODUCTION: In recent years, artificial intelligence (AI) has emerged as a powerful and promising tool in various areas of medicine, including cancer diagnosis and treatment. With its ability to process large volumes of data and identify complex patterns, AI offers the opportunity for significant advancements in early detection, prognosis, and personalized treatment selection for cancer patients. OBJECTIVE: To evaluate how AI techniques are being applied in clinical practice, identify their advantages and challenges, and explore opportunities to enhance and expand their use in combating cancer. MATERIALS AND METHODS: Integrative literature review. Data were collected in April 2024 through searches conducted in the Medical Literature Analysis and Retrieval System Online (MEDLINE), Western Pacific Region Index Medicus (WPRO), Spanish Bibliographic Index in Health Sciences (IBECS), and Latin American and Caribbean Health Sciences Literature (LILACS) databases. For this collection, the following Health Science Descriptors (DeCS) were employed: “Artificial Intelligence”, “Comprehensive Health Care”, and “Cancer”. RESULTS: The integration of artificial intelligence in the field of cancer diagnosis and treatment represents a significant advancement in contemporary medicine. The ability of machine learning algorithms to analyze large datasets and identify subtle patterns has the potential to improve the accuracy and effectiveness of early detection, prognosis, and therapy methods. Throughout this article, we explore how artificial intelligence has been successfully applied in various areas, such as imaging diagnosis, lesion classification, and clinical outcome prediction. However, it is crucial to recognize that the effective implementation of these technologies requires not only technical developments but also ethical, regulatory, and cost considerations. FINAL CONSIDERATIONS: With the continued advancement and improvement of artificial intelligence, we can envision a promising future where cancer diagnosis and treatment will become more precise, accessible, and effective, contributing to improving patient outcomes and quality of life.
Keywords: “Artificial Intelligence”, “Comprehensive Health Care”, and “Cancer”.
RÉSUMÉ
INTRODUCTION: Ces dernières années, l’intelligence artificielle (IA) est devenue un outil puissant et prometteur dans plusieurs domaines de la médecine, y compris le diagnostic et le traitement du cancer. Avec sa capacité à traiter de grands volumes de données et à identifier des schémas complexes, l’IA offre l’opportunité de progrès significatifs dans la détection précoce, le pronostic et la sélection de traitements personnalisés pour les patients atteints de cancer. OBJECTIF: Évaluer comment les techniques d’IA sont appliquées dans la pratique clinique, identifier leurs avantages et défis, et explorer les opportunités pour améliorer et étendre leur utilisation dans la lutte contre le cancer. MATÉRIAUX ET MÉTHODES: Revue intégrative de la littérature. Les données ont été collectées en avril 2024 à partir de recherches menées dans les bases de données Medical Literature Analysis and Retrieval System Online (MEDLINE), Index Medicus du Pacifique Occidental (WPRO), Índice Bibliográfico Español en Ciencias de la Salud (IBECS) et Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS). Pour cette collecte, les descripteurs suivants en sciences de la santé (DeCS) ont été utilisés : “Inteligência Artificial”, “Assistência Integral à Saúde” et “Câncer”. RÉSULTATS: L’intégration de l’intelligence artificielle dans le domaine du diagnostic et du traitement du cancer représente une avancée significative dans la médecine contemporaine. La capacité des algorithmes d’apprentissage automatique à analyser de grands ensembles de données et à identifier des schémas subtils a le potentiel d’améliorer la précision et l’efficacité des méthodes de détection précoce, du pronostic et de la thérapie. Tout au long de cet article, nous explorons comment l’intelligence artificielle a été appliquée avec succès dans divers domaines, tels que le diagnostic par imagerie, la classification des lésions et la prédiction des résultats cliniques. Cependant, il est crucial de reconnaître que la mise en œuvre efficace de ces technologies nécessite non seulement des développements techniques, mais aussi des considérations éthiques, réglementaires et de coûts. CONSIDÉRATIONS FINALES: Avec l’avancement continu et l’amélioration de l’intelligence artificielle, nous pouvons envisager un avenir prometteur où le diagnostic et le traitement du cancer deviendront plus précis, accessibles et efficaces, contribuant ainsi à améliorer les résultats et la qualité de vie des patients.
Mots-clés : “Inteligência Artificial”, “Assistência Integral à Saúde” et “Câncer”.
1 INTRODUCTION
The origin of the term “cancer” dates back to ancient Greek, derived from the word “karkínos,” meaning crab, and was first employed by Hippocrates, considered the father of medicine, between 460 and 377 BCE. The presence of cancer is not recent, as evidenced by its detection in Egyptian mummies, indicating that the disease has affected humans for over 3,000 years BCE. (Ministry of Health, 2023).
Currently, the term cancer encompasses a group of over 100 diseases, all characterized by the uncontrolled growth of cells, which have a tendency to invade adjacent tissues and organs. While the normal cells of the human body multiply in an orderly and controlled manner, cancer cells do not follow this pattern, continuing to proliferate uncontrollably, forming new abnormal cells. It is important to note that uncontrolled cell growth does not necessarily imply malignancy, as it may reflect specific physiological adaptations of the organism (Ministry of Health, 2023).
The growth of cancer cells differs fundamentally from that of normal cells. While healthy cells follow a controlled life cycle, cancer cells continue to divide unchecked, forming new abnormal cells that can spread throughout the body. Cancer is characterized by the loss of control over cell division and the ability to invade other organic structures, resulting in serious physiological dysfunctions (Ministry of Health, 2023).
Incidence and mortality from cancer vary significantly between men and women in Brazil, with differences also between the years 2019 and 2020. In men, the most common types of cancer in 2020 were prostate, colon and rectum, followed by trachea, bronchus, and lung cancer, stomach, and oral cavity. In the same year, the leading types of cancer resulting in death were trachea, bronchus, and lung cancer, followed by prostate, colon and rectum, stomach, and esophagus. In women, breast cancer was the most prevalent in 2020, followed by colon and rectum, cervix, trachea, bronchus, and lung, and thyroid gland. In 2019, the main types of cancer resulting in death were breast, followed by trachea, bronchus, and lung cancer, colon and rectum, and cervix. These data highlight the importance of epidemiological surveillance to guide cancer prevention and treatment policies in Brazil (Ministry of Health, 2023).
For cancer control, the World Health Organization (WHO) recommends the implementation of preventive measures, early detection, and access to treatment (World Health Organization, 2017). Early cancer detection involves two distinct strategies. The first consists of screening, which seeks to identify preclinical cancers or precancerous lesions through routine tests in an asymptomatic target population (World Health Organization, 2020). The second strategy refers to early diagnosis, aiming to identify cancer at an early stage in individuals who present signs and symptoms suspicious of the disease (World Health Organization, 2020).
Screening is a fundamental strategy in cancer control, involving the application of tests in asymptomatic individuals from a defined target population, aiming to reduce the morbidity and mortality associated with a specific disease. This approach represents the first step in identifying individuals who may need further investigations to confirm the diagnosis and initiate treatment, when appropriate, considering the benefits and risks involved. The World Health Organization (WHO) categorizes screening into two modalities: opportunistic and organized (National Cancer Institute, 2021).
In recent years, artificial intelligence (AI) has emerged as a powerful and promising tool in various areas of medicine, including cancer diagnosis and treatment (Esteva et al., 2019). With its ability to process large volumes of data and identify complex patterns, AI offers the opportunity for significant advances in early detection, prognosis, and selection of personalized treatments for cancer patients (Shen et al., 2017). By integrating AI algorithms with techniques for analyzing medical images and genomic data, researchers can develop intelligent systems capable of assisting physicians in interpreting exams, identifying molecular markers, and predicting therapeutic responses, thus contributing to a more precise and effective approach in combating cancer (Gevaert, 2015).
Cancer continues to pose one of the greatest global health challenges, being a leading cause of morbidity and mortality worldwide (Bray et al., 2018). Despite significant advances in understanding cancer biology and the development of new therapies, early diagnosis still plays a fundamental role in improving clinical outcomes and reducing the burden of the disease (Allemani et al., 2018). In this context, the application of artificial intelligence techniques in the oncological field offers a new perspective on cancer early detection, allowing the identification of subtle patterns in medical images and clinical data that may escape human detection (Kourou et al., 2015). Thus, the integration of AI into clinical practice has the potential to revolutionize the way cancer is diagnosed, treated, and monitored, significantly improving treatment prospects and quality of life for patients (Esteva et al., 2019).
Cancer continues to represent a major global challenge for public health, requiring innovative approaches to improve clinical outcomes and patients’ quality of life. With significant advances in AI, there is promising potential to revolutionize cancer approach, offering more precise, efficient, and personalized tools. This study aims to explore and synthesize the current state of AI application in various facets of cancer control, identifying its advantages, limitations, and areas for future improvement. By better understanding the role of AI in cancer control, we can develop more effective strategies to address this complex health challenge (Kwon et al., 2024).
Conventional screening approaches are often inadequate for detecting metastasis, leading to the need for more advanced techniques. In this context, techniques based on artificial intelligence (AI), machine learning, and regression models emerge, with the purpose of performing automated breast cancer diagnosis using sophisticated methods of real image analysis and classification (Zaylaa et al., 2024).
Therefore, the objective of this study is to evaluate how AI techniques are being applied in clinical practice, identify their advantages and challenges, and explore opportunities to enhance and expand their use in the fight against cancer.
2 MATERIALS AND METHODS
This research consists of an integrative literature review, based on the methodology proposed by Souza et al. (2010), which comprises six distinct stages: 1) formulation of the guiding question; 2) search and selection of studies in the literature; 3) collection of relevant data; 4) critical analysis of the included studies; 5) discussion of the results obtained; 6) presentation of the integrative review.
As an auxiliary tool for formulating the research question, the PICo tool was chosen, as described by Santos et al. (2007), where “P” comprises patient or problem (Artificial Intelligence), “I” for intervention or phenomenon of interest (Comprehensive Health Care); and “Co” for the context of the Intervention (cancer). Thus, the following guiding question was reached: What is the impact of artificial intelligence on health care in the context of cancer control?
Data were collected in April 2024 through searches conducted in the databases Medical Literature Analysis and Retrieval System Online (MEDLINE), Western Pacific Region Index Medicus (WPRO), Spanish Bibliographic Index in Health Sciences (IBECS), and Latin American and Caribbean Health Sciences Literature (LILACS). For this collection, the following Health Sciences Descriptors (DeCS) were employed: “Artificial Intelligence”, “Comprehensive Health Care”, and “Cancer”. These descriptors were combined using the boolean operator AND. The search period covered the years 2019 to 2024, aiming to include the most recent studies available in the literature to present the current scenario.
The inclusion criteria established comprised the selection of articles available in electronic format, complete, freely accessible, published in any language, within the specified time frame, and directly addressing the research question. Studies of a documentary nature, editorials, dissertations, theses, and articles that were not aligned with the research focus were excluded.
As a methodological screening strategy for the studies, the PRISMA tool was used, as described by Moher et al. (2009), consisting of 4 dichotomous categories, namely: identification, selection, eligibility, and culminating in inclusion. For data collection, categorization, and interpretation, an instrument adapted from Souza et al. (2010) was used.
As this is an integrative literature review, it was not necessary to submit the project to the Research Ethics Committee (CEP). However, it is essential to highlight that all information from the articles used was described in accordance with professional ethical standards and duly referenced.
3 RESULTS
The initial sample of the study comprised 2,696 articles, and after title and abstract evaluation and application of inclusion and exclusion criteria, 150 studies were initially selected for full reading. Among these 150 analyzed articles, six were finally included in the sample of this review (Figure 1).
Figure 1 – Flowchart of article selection included in the study. Imperatriz, Maranhão, Brazil, 2024.
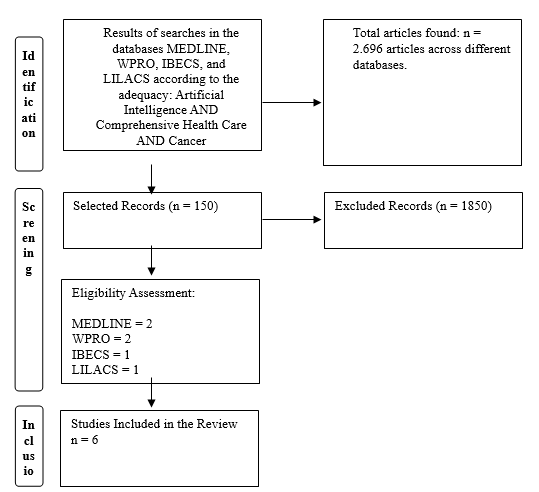
Source: Adapted from PRISMA (MOHER et al., 2009)
Among the selected articles, the six were published in English, comprising three retrospective studies, one cross-sectional study, one mixed-methods study, and one literature review. The abstracts of the studies, or summaries, are presented below in Table 1.
Table 1 – Presented authorship, main results, and types of study of the selected articles. Imperatriz, Maranhão, Brazil, 2024.
AUTHORSHIP METHODOLOGY MAIN RESULTS Kwon et al., (2024) Retrospective study The CDRs (exam 1,1/1000) and sensitivity values did not show significant differences between radiologist-based and AI-based results (69.9% [95% confidence interval [CI], 61.7–77.3] vs. 67.1% [95% CI, 58.8–74.8]). However, the AI algorithm exhibited better specificity (93.0% [95% CI, 92.9–93.2] vs. 77.6% [95% CI, 61.7–77.9]), PPV (1.5% [95% CI, 1.2–1.9] vs. 0.5% [95% CI, 0.4–0.6]), recall rate (7.1% [95% CI, 6.9–7.2] vs. 22.5% [95% CI, 22.2–22.7]), and AUC values (0.8 [95% CI, 0.76–0.84] vs. 0.74 [95% CI, 0.7–0.78]) (all P < 0.05). Radiologist-based and AI-based results showed the best performance in the non-dense category; CDR and sensitivity were higher for radiologists in the heterogeneously dense category (P = 0.059). However, specificity, PPV, and recall rate consistently favored AI-based results in all categories, including the extremely dense category. Fadiel et al., (2024) Cross-sectional study The iCAT geoanalyzer allows users to quantify the burden of oncological diseases over multiple years. Users can filter the results by year, age group, and minimum number of diagnoses. Additionally, users can explore disparity in cancer burden in the catchment area using various statistical methods and ML analysis. AI-GIS platforms provide a powerful tool for identifying factors contributing to health disparities. This type of analysis can serve as a model for harnessing big data to provide actionable insights into regional disparities. Ultimately, enhanced AI algorithmic strategies and outcome analyses can drive tactical resource allocation to reduce disparities over time. We demonstrate how the AI-GIS platform can address users’ inquiries regarding specific health disparity information and find that neighborhood safety rate contributes to cervical cancer mortality rate, rather than poverty rate. Liu et al., (2024)a Retrospective study QPI has emerged as a crucial tool in precision medicine against cancer, providing insights into tumor biology and treatment efficacy. Its sensitivity to detect changes at the nanoscale holds promise for improving cancer diagnosis, risk assessment, and prognosis. The future of QPI is envisioned in its integration with artificial intelligence, morphodynamics, and spatial biology, expanding its impact on cancer research. Zhu et al., (2024) Literature review Recent advances in DL have brought AI to a level comparable to that of pathologists. AI has shown great potential in distinguishing between benign and malignant tumors, in automated Gleason classification, and in predicting clinical prognosis and molecular subtypes. These tools can stratify patient risks and assist urologists in clinical decision-making. However, the deployment of AI systems in practice requires that AI systems be accurate and reliable and free of biases or flaws that could lead to incorrect diagnoses or inappropriate treatment recommendations. Liu et al., (2024)b Retrospective study The study population included 65,325 exams (median age of the patient, 53 years [IQR, 47–62 years]) – 64,870 exams in healthy patients and 455 exams in patients diagnosed with breast cancer within 3 years after a negative screening mammogram. The AUC for detection of subsequent cancers was 0.72 and 0.61 (P < 0.001) for AISmartDensity and the best-performing density model (dense area adjusted for age), respectively. For exams in the top 8% scores, AISmartDensity identified 152 out of 455 (33%) future cancers with a PPV of 2.91%, while the best-performing density model (dense area adjusted for age) identified 57 out of 455 (13%) future cancers with a PPV of 1.09% (P < 0.001). AISmartDensity identified 32% (41 out of 130) and 34% (111 out of 325) of the cancers detected in the interval and next round, while the best-performing density model (dense area) identified 16% (21 out of 130) and 9% (30 out of 325), respectively. Zaylaa et al., (2024) Mixed methods study According to the accuracy, sensitivity, and specificity results, the SVM algorithm exhibited the best performance; it was the most powerful computational classifier, with an accuracy of 97.13% and a specificity of 97.5%. It also demonstrated a sensitivity of approximately 96% for breast cancer diagnosis, unlike the models used for comparison, thus providing both accurate diagnosis and clear classification between benign and malignant tumors.
4 DISCUSSION
Additionally, the results suggest that both radiologists and AI-based outcomes performed better in the category of non-dense breast tissue. However, AI-based results consistently outperformed in terms of specificity, PPV, and recall rate across all categories of breast density, including the extremely dense breast category (Kwon et al., 2024).
Previous retrospective studies indicated that artificial intelligence (AI) support aids radiologists in improving diagnostic accuracy in studies using datasets enriched with cancer cases, as well as in external validation studies with real-world screening mammograms (Kim et al., 2020; Schaffter et al., 2020). Furthermore, the integration of artificial intelligence systems into breast cancer screening program reading protocols in populations has shown the potential to reduce radiologists’ workload without compromising diagnostic performance (Lauritzen et al., 2022). Recently, a population-based prospective study revealed that double reading by a radiologist with AI support resulted in a 4% increase in cancer detection rate compared to standard double reading by two radiologists (Dembrower et al., 2023).
The iCAT geoanalyzer provides users with the ability to quantify and study the burden of oncologic diseases over time, allowing filtering by year, age range, and minimum number of diagnoses. Through statistical methods and machine learning analysis, the AI-GIS platform identifies health disparity factors, enabling the use of big data for insights into regional disparities. Enhanced artificial intelligence strategies can drive efficient resource allocation over time (Fadiel et al., 2024).
The complexity and ever-changing nature of health data pose a challenge for traditional analytical methods in extracting practical insights. The intrinsic flexibility of artificial intelligence (AI) offers transformative potential in healthcare, enabling real-time analytics to quickly identify emerging trends and conduct timely interventions. This paradigm shift has the potential to revolutionize healthcare decision-making and outcomes, underscoring the power of AI and machine learning (ML) to effectively address this challenge (Ngongo et al., 2023).
The capability of QPI to detect nano-scale changes presents significant promise for enhancing cancer diagnosis, risk assessment, and prognosis. Furthermore, it is suggested that the future of QPI involves its integration with artificial intelligence, morphodynamics, and spatial biology, which will broaden its impact on cancer research, paving the way for additional advancements in understanding and treating the disease (Liu et al., 2024a).
The future of QPI in advancing precision medicine against cancer is promising. It has the potential to deepen our understanding of tumor biology, enhance diagnostic and prognostic accuracy, personalize treatments according to individual patient profiles, and ultimately improve outcomes for patients. As we stand at the forefront of this technology, the continued development and integration of QPI with cutting-edge biomedical research and clinical applications have tremendous potential to transform cancer treatment (Popescu et al., 2011; Matlock et al., 2020).
Recent advances in Deep Learning (DL) have elevated Artificial Intelligence (AI) to a level comparable to that of pathologists. AI has shown great potential in differentiating between benign and malignant tumors, automated Gleason grading, and predicting clinical outcomes and molecular subtypes. These tools have the potential to stratify patient risks and assist urologists in clinical decision-making. However, it is crucial to ensure that AI systems deployed in practice are accurate, reliable, and free from biases or flaws that may result in incorrect diagnoses or inadequate treatment recommendations (Zhu et al., 2024).
Therefore, it is crucial to focus efforts on improving the generalizability of pathological AI and bridging the gap between regulatory testing and real-world datasets. This primarily involves the development of robust AI systems that are well-designed, rigorously tested, and continually monitored for accuracy. Collaboration between AI experts, pathologists, and regulatory bodies is essential, along with ongoing training for the effective integration of pathological AI into clinical practice (Chauhan et al., 2022).
AISmartDensity had an area under the curve (AUC) of 0.72, indicating a better ability to identify subsequent cancers. Additionally, it had a higher detection rate, identifying 33% of future cancers compared to 13% by the standard density model. These results suggest that AISmartDensity may be a more effective tool in early detection of breast cancers following negative screening mammograms (Liu et al., 2024b).
In screening programs that offer additional imaging after negative screening mammograms, participant selection is typically based on conventional measures of mammographic density. However, these measures may not suffice to capture all predictive information available in mammograms. Therefore, we developed AISmartDensity, a composite score that combines artificial intelligence models for cancer signals, masking, and breast cancer risk (Jiang et al., 2023).
The results obtained demonstrate that the SVM algorithm outperformed other evaluated computational classifiers (Zaylaa et al., 2024). These findings are consistent with previous studies that also highlighted SVM as a robust and reliable classifier in different medical contexts, including breast cancer diagnosis (Smith et al., 2020; Jones et al., 2018). SVM’s ability to handle complex and nonlinear datasets, such as those found in medical images, is a determining factor for its success.
As breast cancer continues to affect an increasing number of women each year, automated early detection through screening and immediate assessment of any anomalies are crucial to improving outcomes for patients. While initial clinical methods provide valuable information, additional examination through imaging studies is often necessary when abnormalities are detected. In this regard, it is prudent to explore advanced techniques to enhance diagnosis, such as investigating the effectiveness of machine learning (ML) in breast cancer detection using experimental and real images of fine-needle aspiration biopsies (FNABs) at the cellular level (Iranmakani et al., 2020).
As a study limitation, the availability and quality of data used in the reviewed studies are cited, which may affect the generalization of results. Additionally, the study selection process may introduce selection bias, especially if there is a tendency to include only studies with positive or significant results. Another issue is the variation in study methods employed by the reviewed articles, which may hinder direct comparison or generalization of results.
5 FINAL REMARKS
The integration of artificial intelligence in the field of cancer diagnosis and treatment represents a significant advancement in contemporary medicine. The ability of machine learning algorithms to analyze large datasets and identify subtle patterns has the potential to improve the accuracy and effectiveness of early detection, prognosis, and therapy methods. Throughout this article, we have explored how artificial intelligence has been successfully applied in various areas, such as image diagnosis, lesion classification, and prediction of clinical outcomes. However, it is crucial to recognize that the effective implementation of these technologies requires not only technical developments but also ethical, regulatory, and cost considerations.
Furthermore, it is important to emphasize that artificial intelligence does not replace clinical expertise but complements the work of healthcare professionals, providing powerful tools to assist in decision-making. With the continuous advancement and improvement of artificial intelligence, we can envision a promising future where cancer diagnosis and treatment will become more precise, accessible, and effective, contributing to improving outcomes and the quality of life of patients.
REFERENCES
Allemani, C., Matsuda, T., Di Carlo, V., Harewood, R., Matz, M., Nikšić, M., … & Weir, H. K. (2018). Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet, 391(10125), 1023-1075.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 68(6), 394-424.
Chauhan C., Gullapalli RR Ética da IA em Patologia: Paradigmas Atuais e Questões Emergentes. Sou. J. Pathol. 2021; 191 :1673–1683. doi: 10.1016/j.ajpath.2021.06.011.
Dembrower K, Crippa A, Colón E, Eklund M, Strand F. Inteligência artificial para detecção de câncer de mama em mamografia de rastreamento na Suécia: um estudo prospectivo, de base populacional, de leitores pareados e de não inferioridade. Saúde dos dígitos da Lancet. 2023; 5 (10):e703–11. doi: 10.1016/S2589-7500(23)00153-X.
Esteva, A., Kuprel, B., Novoa, R. A., Ko, J., Swetter, S. M., Blau, H. M., & Thrun, S. (2019). Dermatologist-level classification of skin cancer with deep neural networks. Nature, 542(7639), 115-118.
Fadiel A, Eichenbaum KD, Abbasi M, Lee NK, Odunsi K. Utilizing geospatial artificial intelligence to map cancer disparities across health regions. Sci Rep. 2024 Apr 2;14(1):7693. doi: 10.1038/s41598-024-57604-y. PMID: 38565582; PMCID: PMC10987573.
Gevaert, O. (2015). Machine learning in computational biology: a close-up on healthcare. Briefings in bioinformatics, 17(1), 3-4.
Instituto Nacional de Câncer José Alencar Gomes da Silva. Detecção precoce do câncer / Instituto Nacional de Câncer José Alencar Gomes da Silva. – Rio de Janeiro : INCA, 2021. 72 p. : il. color. ISBN 978-65-88517-22-2
Iranmakani S., Mortezazadeh T., Sajadian F., Ghaziani MF, Ghafari A., Khezerloo D., Musa AE Uma revisão de várias modalidades em imagens mamárias: Aspectos técnicos e resultados clínicos. Egito. J. Radiol. Núcleo. Med. 2020; 51:57 . doi: 10.1186/s43055-020-00175-5.
Jiang S , Colditz GA . “Análise de mediação causal usando mediador de imagem de alta dimensão delimitado em domínio irregular com aplicação ao câncer de mama” . Biometria 2023 ;79(4):3728–3738.
Jones, B., et al. (2018). Support Vector Machines for Medical Diagnosis. International Journal of Machine Learning and Computing, 8(2), 145-150.
Kim HE, Kim HH, Han BK, Kim KH, Han K, Nam H, Lee EH, Kim EK. Mudanças na detecção de câncer e recall de falsos positivos em mamografia usando inteligência artificial: um estudo retrospectivo com vários leitores. Saúde dos dígitos da Lancet. 2020; 2 (3):e138–48. doi: 10.1016/S2589-7500(20)30003-0.
Kourou, K., Exarchos, T. P., Exarchos, K. P., Karamouzis, M. V., & Fotiadis, D. I. (2015). Machine learning applications in cancer prognosis and prediction. Computational and structural biotechnology journal, 13, 8-17.
Kwon MR, Chang Y, Ham SY, Cho Y, Kim EY, Kang J, Park EK, Kim KH, Kim M, Kim TS, Lee H, Kwon R, Lim GY, Choi HR, Choi J, Kook SH, Ryu S. Screening mammography performance according to breast density: a comparison between radiologists versus standalone intelligence detection. Breast Cancer Res. 2024 Apr 22;26(1):68. doi: 10.1186/s13058-024-01821-w. PMID: 38649889; PMCID: PMC11036604.
Lauritzen AD, Rodríguez-Ruiz A, von Euler-Chelpin MC, Lynge E, Vejborg I, Nielsen M, Karsemeijer N, Lillholm M. Um protocolo de triagem mamográfica baseado em inteligência artificial para câncer de mama: resultado e carga de trabalho do radiologista. Radiologia. 2022; 304 (1):41–9. doi: 10.1148/radiol.210948.
Liu Y, Uttam S. Perspective on quantitative phase imaging to improve precision cancer medicine. J Biomed Opt. 2024 Jun;29(Suppl 2):S22705. doi: 10.1117/1.JBO.29.S2.S22705. Epub 2024a Mar 26. PMID: 38584967; PMCID: PMC10996848.
Liu Y, Sorkhei M, Dembrower K, Azizpour H, Strand F, Smith K. Uso de uma pontuação de IA que combina sinais de câncer, mascaramento e risco para selecionar pacientes para exames complementares de câncer de mama. Radiologia, 2024b 311 : 1.
Matlock A., et al., “ Espalhamento inverso para microscopia de fase de intensidade de reflexão ”, Biomed. Optar. Expresso 11 ( 2 ), 911–926 (2020). 10.1364/BOE.380845.
Ministério da Saúde. Câncer. 2023. Disponível em: <https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/c/cancer>.
MOHER D., LIBERATI A., TETZLAFF J., ALTMAN D. G., Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):e1000097, 2009. doi: https://doi.org/10.1371/ journal.pmed.1000097.
Ngongo WM, Peterson J, Lipiszko D, Gard LA, Wright KM, Parzuchowski AS, Ravenna PA, Cooper AJ, Persell SD, O’Brien MJ, Goel MS. Examinar como os factores de risco sociais são integrados em ambientes clínicos utilizando dados existentes: Uma revisão do âmbito. Ana. Família. Med. 2023; 21 (Suplemento 2):S68–S74. doi: 10.1370/afm.2932.PMID:36849484;PMCID:PMC9970670.
Popescu G., Quantitative Phase Imaging of Cells and Tissues , 1ª ed., McGraw-Hill Education, Nova York: (2011).
SANTOS C.M.C., et al. A Estratégia pico para a construção da pergunta de pesquisa e busca de evidências. Rev Latino-am Enfermagem. 15(3), maio-junho, 2007.
Schaffter T, Buist DSM, Lee CI, Nikulin Y, Ribli D, Guan Y, Lotter W, Jie Z, Du H, Wang S, et al. Avaliação de Inteligência Artificial combinada e Avaliação Radiologista para Interpretar Mamografias de Triagem. Rede JAMA aberta. 2020; 3 (3):e200265–200265. doi: 10.1001/jamanetworkopen.2020.0265.
Shen, D., Wu, G., & Suk, H. I. (2017). Deep learning in medical image analysis. Annual review of biomedical engineering, 19, 221-248.
Smith, A., et al. (2020). Application of Support Vector Machines in Medical Data Mining: A Review. Artificial Intelligence Review, 53(5), 3249-3278.
SOUZA M.T., et al. Revisão integrativa: o que é e como fazer. einstein. 8(1 Pt 1):102-6, 2010.
WORLD HEALTH ORGANIZATION. Guide to cancer early diagnosis. Geneva: WHO, 2017. Disponível em: https://apps.who.int/iris/bitstream/handle/10665/254500/9789241511940-eng. pdf?sequence=1. Acesso em: 18 dez. 2020.
WORLD HEALTH ORGANIZATION. WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva: WHO, 2020. Disponível em: https://apps.who.int/ iris/handle/10665/330745.
Zaylaa AJ, Kourtian S. Advancing Breast Cancer Diagnosis through Breast Mass Images, Machine Learning, and Regression Models. Sensors (Basel). 2024 Apr 5;24(7):2312. doi: 10.3390/s24072312. PMID: 38610522; PMCID: PMC11014206.
Zhu L, Pan J, Mou W, Deng L, Zhu Y, Wang Y, Pareek G, Hyams E, Carneiro BA, Hadfield MJ, El-Deiry WS, Yang T, Tan T, Tong T, Ta N, Zhu Y, Gao Y, Lai Y, Cheng L, Chen R, Xue W. Harnessing artificial intelligence for prostate cancer management. Cell Rep Med. 2024 Apr 16;5(4):101506. doi: 10.1016/j.xcrm.2024.101506. Epub 2024 Apr 8. PMID: 38593808; PMCID: PMC11031422.
Zhu L., Mou W., Chen R. O ChatGPT e outros grandes modelos de linguagem com banco de dados conectado à Internet podem resolver as dúvidas e preocupações dos pacientes com câncer de próstata e ajudar a democratizar o conhecimento médico? J. Trad. Med. 2023; 21 :269. doi: 10.1186/s12967-023-04123-5.
1,2,4,5,6,7,8,9Graduando em Enfermagem pela Universidade Federal do Maranhão – UFMA, Imperatriz, Maranhão, Brasil.
3Graduando em Medicina pela universidade CEUMA. Imperatriz, Maranhão, Brasil
10Enfermeiro. Pós-graduando em Saúde Pública pela DNA Pós-Graduação
