REGISTRO DOI: 10.5281/zenodo.12611384
Dayane Mirelle de Arruda Pereira¹
Renata Duarte Batista ¹
Ana lívia Nóbrega Rolim¹
Juliana Barbosa Cabral¹
Andresa Pinto de Araújo¹
Rute da Silva¹
Yasmin Bruna Monteiro Negreiros Lira¹
Jamylle Yngrid Chagas do Nascimento¹
Enaianny Ribeiro dos Santos Frankenberger²
Regina Amélia Gonzaga Trajano Nunes³
Allisson Francisco de Morais⁴
Cibele Lopes de Santana Ramalho⁴
Introduction: Malaria, an infectious disease predominantly endemic in tropical and subtropical regions globally, arises from the rapid proliferation of the Plasmodium genus within the bloodstream, culminating in severe clinical manifestations. Notably, regions with elevated incidences of sickle cell disease (SCD) showcase a marked 90% reduction in the susceptibility to Plasmodium falciparum malaria among affected children in sub-Saharan Africa.Objective: To examine the prevalence of sickle cell anemia in children and its association with the acquisition of resistance against Plasmodium falciparum malaria. Methodology:Methodology: This study employs an integrative review with a narrative approach, utilizing electronic databases such as PUBMED, LILACS, Medline, among others. Boolean operators “AND” were used with “sickle cell” and “Malaria Falciparum”, while “OR” was used with “Plasmodium falciparum” and “Hemoglobin Sickle”. Articles included were fully available in Portuguese and English languages, excluding duplicates and those not addressing the topic, resulting in 36 articles identified. Out of these, 18 were selected for the study, covering the period from (2013 – 2024). Results and Discussion:The analyzed studies predominantly investigate the clinical manifestations and prevalence of malaria in pediatric patients with sickle cell anemia, exploring these aspects in conjunction with the molecular mechanisms of hemoglobin S (HbS) against the protozoan. The literature highlights a significant decrease in malaria prevalence among this demographic, associated with lower parasite loads that mitigate the severity of the disease. Additionally, certain studies suggest that malaria may exacerbate existing anemia in individuals with sickle cell disease, potentially worsening morbidity and mortality outcomes. Conclusion: The data clarifies that hemoglobin S (HbS) provides partial protection against severe malaria in pediatric populations, albeit with certain limitations. Therefore, endemic regions require additional antimalarial prophylaxis and targeted interventions to reduce the incidence and mortality of malaria, regardless of sickle cell disease status.
Keywords: Malaria Falciparum; Sickle cell; Child; Hemoglobin SC Disease; Clinical Epidemiology
1 INTRODUCTION:
Malaria represents a parasitic disease of significant importance in regions of Africa and socioeconomically challenged nations, posing a critical public health concern (CHIMUCO, 2022). It is caused by parasites of the Plasmodium genus, including three species that infect humans: P. falciparum, P. vivax, and P. malariae. Transmission occurs through the bite of Anopheles mosquitoes, which host both the sexual and asexual phases of the parasite’s life cycle (SATO, 2021).
The biological cycle commences with the inoculation of sporozoites by the mosquito vector into the epidermis, where they replicate within hepatocytes—specifically liver cells—over approximately 8-9 days, generating numerous merozoites, which are progeny parasites. Subsequently, these merozoites rupture erythrocytes, initiating the phase known as blood schizogony. This stage involves unchecked replication followed by the lysis of infected erythrocytes and invasion of additional erythrocytes (MINISTERIO DA SAUDE, 2020). Transmission can occur through blood transfusion, vertical transmission from mother to fetus during pregnancy, and incidents involving puncturing injuries with biological material ( REAL, 2021).
Sickle cell hemoglobin (HbS) is an autosomal recessive hemoglobinopathy resulting from mutations in the β-globin gene, causing morphological alterations in erythrocytes that take on a sickle-shaped form. This mutation transforms normal hemoglobin (HbA) into the abnormal HbS (ELENDU, 2023). Consequently, these altered erythrocytes adhere to vascular endothelium, leading to obstruction and resulting in vaso-occlusion, thrombosis, and fibrosis in various organs. Serious health complications ensue, such as painful crises and chronic organ damage (CONRAN,2021).
In sub-Saharan Africa, an endemic region for P. falciparum malaria with significant mortality rates, approximately 80% of individuals are born with sickle cell anemia. Studies explore the evolutionary dynamics of these populations, investigating the genetic variant (HbS) as a potential adaptive response against malaria(AMBROSE,2020).
Furthermore, sickle cell trait (HbAS), in its heterozygous form, confers significant resistance to malaria, although the precise biological mechanism remains incompletely understood with several hypotheses proposed. This resistance is believed to stem from the physical-chemical properties of erythrocytes carrying HbAS, which influence the invasion, growth, and development of P. falciparum, potentially through immunological processes that hinder parasite survival. Infected erythrocytes may undergo intravascular sickling, leading to premature destruction and a reduction in parasite load (CHIMUCO, 2022). Consequently, this review aims to analyze the prevalence of malaria resistance among children with sickle cell anemia due to their HbAS status.
2 METHODOLOGY:
This is an integrative review employing a narrative approach, utilizing electronic databases such as PUBMED, LILACS, Medline, among others. The primary descriptors used were Malaria Falciparum, Sickle cell, Child, Hemoglobin Sickle, Clinical Epidemiology, combined with Boolean operators “AND” and “OR.” The study was conducted during April and May, selecting articles from the period between 2013 and 2024, and including publications in Portuguese, English, and Spanish.
Inclusion criteria focused on articles presenting scientific evidence related to clinical aspects, particularly the prevalence of sickle cell anemia in children and its association with biochemical defense mechanisms against malaria involving hemoglobin S. Exclusion criteria encompassed manuals, dissertations, theses, articles lacking abstracts, studies not directly addressing the topic, superficial or tangential themes, and publications predating 2013. Articles underwent initial screening based on title assessment using Boolean operators “AND” and “OR,” selecting those with pertinent abstracts and potential findings aligned with the study’s objective.
Table 1: illustrates the flowchart depicting the article selection strategy according to pre-established eligibility criteria
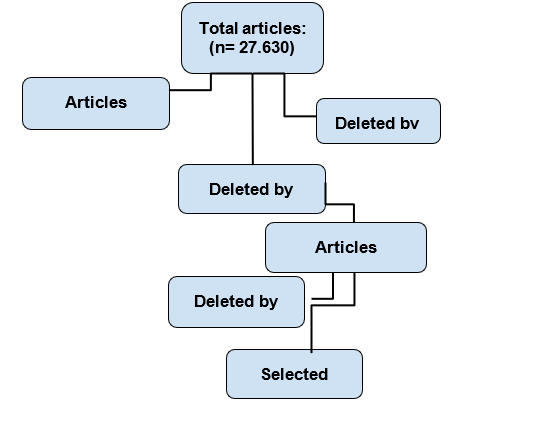
3 RESULTS:
A total of 1226 articles related to the topic were identified, adhering to eligibility criteria. Four articles focusing on the prevalence of children with sickle cell anemia in tropical and subtropical regions as an evolutionary factor in malaria resistance were located. These articles met inclusion and exclusion criteria, proving pivotal to the research.
Table 2 presents an analysis of the articles including author and publication year, objectives, methodological study design, sample representativeness, results, and conclusion. The articles are classified as original research studies (n=5), comprising 1 retrospective analysis, 2 clinical trials detailing study design, sample characteristics, and outcomes, and 1 cohort study along with 2 randomized trials.”
Table 2: Epidemiological profile of children with sickle cell anemia related to malaria
Ngou, et.al,2023 To trace the protection offered by sickle cell traits against malaria in asymptomatic children in endemic areas. Clinical Trial 1557 children, of which 20.16% had the HbAS trait. The prevalence of P. falciparum gametocytes was 18.47% in HbAS children, indicating resistance to the parasite. Individuals with HbAS showed protection against severe malaria through cytoadhesion of infected erythrocytes to the endothelium, reducing parasitic load. The presence of the sickle cell trait (HbAS) offers protection against severe malaria, demonstrated by reduced prevalence of gametocytes and decreased cytoadhesion. Eleonore, et al. 2019 To assess the prevalence of malaria in endemic areas and its impact on individuals with the sickle cell trait. Cohort Study Admission records of children aged 1 to 18 with confirmed malaria diagnosis. Children with SCD had lower malaria prevalence (23.5%) compared to those without SCD (44.9%). Mortality was lower in individuals with SCD (20.4%) compared to those without SCD (35.4%). The sickle cell disease population exhibits lower prevalence and mortality from malaria, suggesting a survival advantage. Uyoga, et.al.2022 To explore the relationship between sickle cell anemia, P. falciparum malaria, and infection burden in children with severe anemia. Open-label, Multicenter, Factorial, Randomized Clinical Trial 3483 children (30% with SCD, 78% without SCD) aged 2 months to 12 years. The prevalence of parasitic load was lower in children with SCD. Children with sickle cell disease (SCD) benefit from inherent defense against severe malaria. However, minimal parasitic loads fail to mitigate severe anemic crises, underscoring the potential fatality of even low parasitic burdens. Oppong, et.al., 20 The occurrence of sickle cell disease and malaria infection among children aged 1 to 12 years in the Volta region. cross-sectional survey A total of 938 children aged 1-12 years from three districts in the Volta region were included in a multi-stage sampling process. A prevalence of 16% for sickle cell anemia was observed, contrasting with the global prevalence of 2.0%. Among genotypes, HbSF showed the highest prevalence. Microscopic parasitemia for P. falciparum was detected in 5.5% of children, with a submicroscopic prevalence of 14.2% via PCR. Sickle cell diseases are associated with submicroscopic parasitemia infection as well as anemia compared to normal genotypes. Deme/ly, et.al., 2022 Study the epidemiological characteristics, diagnosis, and progression of malaria in children with sickle cell disease (SCD). retrospective, descriptive, and analytical 3,773 children were analyzed in outpatient care for sickle cell disease among patients under 16 years old over a span of 3 years. Among them, 21 presented with malaria, but only 14 of them were evaluated. In the hospital, a frequency of 0.5-7% cases per year was observed for the association of sickle cell disease with malaria. Among the 14 patients homozygous for SS evaluated, 13 tested positive for P. falciparum. All patients exhibited anemia with hemoglobin levels of 7.74 g/dl. Additionally, 63.6% showed thrombocytosis, and bone vaso-occlusion due to sickle cell disease (SCD) was also observed. . The study evaluated predominantly male children, all homozygous for SS, where fever manifested clinically as malaria and vaso-occlusive crises of sickle cell disease (SCD). However, malaria should be considered severe in cases of chronic anemia.
4 DISCUSSION:
Sickle cell anemia is an autosomal recessive disorder primarily characterized by sickle cell disease, wherein red blood cells assume a distinctive “sickle” shape. These altered cells exhibit increased rigidity and adhesiveness, which can lead to compromised blood flow due to potential blockages (Elendu, 2023). Clinical symptoms of sickle cell disease typically appear in infancy, as early as 6 months of age, and may include chronic pain episodes, swelling in the hands and feet, recurrent infections, retinal complications, and growth retardation (Mangla, 2023).
4.1 Parasitic Infection by Plasmodium falciparum:
Plasmodium falciparum is a subspecies of malaria endemic primarily to sub-Saharan Africa, with additional foci in North and South Korea, Haiti, Argentina, Turkey, Iraq, and other regions. In 2019, an estimated 241 million malaria cases were reported globally, with Africa bearing 95% of the burden, resulting in approximately 627,000 malaria-related deaths. Children under the age of 5 accounted for a significant proportion of these fatalities (OMS,2022).
The biological cycle of P. Falciparum species commences when female Anopheles mosquitoes ingest blood containing gametocytes from an infected individual. Following ingestion, gametocytes undergo sexual reproduction within the mosquito’s midgut over a period of approximately 1-2 weeks. This process results in the production of infectious sporozoites, which migrate to the mosquito’s salivary glands. Upon subsequent blood feeding by the mosquito, these sporozoites are injected into a new host, where they swiftly migrate to the liver and invade hepatocytes (SATO, 2021).
These parasites undergo maturation into tissue schizonts within liver cells, generating between 10,000 and 30,000 merozoites. After 1-3 weeks, hepatocyte rupture releases these merozoites into the bloodstream. This biological stage enables invasion of red blood cells, where the parasite transforms into a trophozoite. The trophozoite matures into erythrocytic schizonts, producing new merozoites that subsequently rupture red blood cells, releasing them into the plasma and perpetuating the cycle (MARIE, 2022).
Malaria caused by P. falciparum results in the most severe manifestation of the disease due to microvascular effects, causing abnormal hemodynamics and a spectrum of symptoms. These include seizures, jaundice, diarrhea, retinal hemorrhages, severe thrombocytopenia, renal failure, vascular obstruction by parasitized red blood cells, hemoglobinuria, and hemoglobinemia. These symptoms indicate intravascular hemolysis and the potential for hemoglobinuric fever (MINISTERIO DA SAUDE, 2020).
Figure 1- Life cycle of Plasmodium
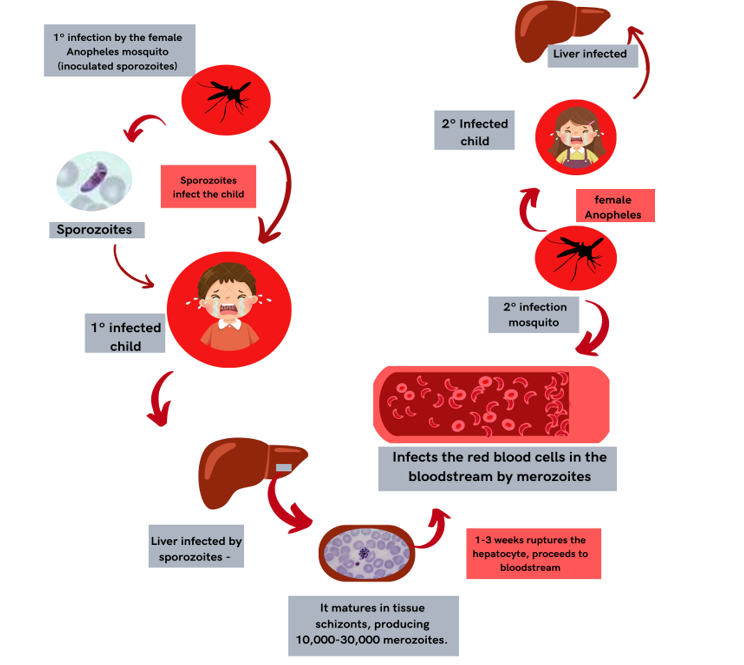
FONTE: Autores (2024).
4.2 Biochemical Mechanisms of Sickle Cell Anemia( HbS) Resistant to Malaria:
Malaria has exerted evolutionary pressure on the human genome, leading to the development of multiple genetic polymorphisms that confer protection against severe forms of the disease (OPPONG,2020).
One notable example is Sickle Cell Hemoglobin (HbS), which has been demonstrated to reduce parasitemia and mitigate clinical malaria compared to Normal Hemoglobin (HbAA). However, individuals with HbS are still susceptible to severe anemic crises, particularly in settings where transfusion services are inaccessible (UYAGA, 2022).
Another study reports that children with sickle cell trait (SCT) exhibit lower mortality compared to those without SCT when infected with malaria (Eleonore, 2020). Mosquitoes fed on blood containing hemoglobin AS (HbAS) showed reduced transmission efficiency due to the membrane properties of erythrocytes that can impact the maturation of gametocytes – the infective form of Plasmodium – in circulating red blood cells (NGOY, 2023).
According to a recent clinical trial study, the sickle cell trait was identified in 314 children, accounting for 20.16% of the cohort. Among these children, 18.47% demonstrated resistance to malaria and protection against severe malaria (NHOU, 2023).
It is proposed that HbS induces biochemical changes in erythrocytes that influence the metabolism and growth of the malaria parasite. Specifically, the accelerated auto-oxidation in HbS leads to a high rate of free heme, which can stimulate the production of Heme Oxygenase-1 (HO-1). HO-1 is an enzyme responsible for converting protoporphyrin IX into biliverdin, releasing iron (Fe), and generating carbon monoxide (CO). This process contributes to host tolerance during severe diseases such as cerebral malaria (ERIDANI,2011).
Furthermore, these biochemical alterations interfere with the formation of the actin cytoskeleton, modifying the surface membrane of infected erythrocytes. This alteration reduces cytoadherence and subsequently decreases inflammation in the pathogenesis of severe malaria. However, it may also result in the uptake of infected red blood cells by the spleen (CHIMUCO,2022).
In the spleen, heightened antigen presentation triggers enhanced immune responses, including the production of antibodies targeting PfEMP1 and other surface proteins. This biochemical response plays a crucial role in protecting against high parasitic loads by disrupting cytoadherence, promoting opsonization, and facilitating the phagocytosis of infected erythrocytes. Consequently, children with HbAS are shielded from both uncomplicated and severe malaria (CONRAN,2021).
Studies have highlighted sickle cell anemia as a protective factor against Plasmodium falciparum, comparing children with abnormal HbS to those with normal HbA. Biochemical and immunological mechanisms play a crucial role in decreasing parasitic loads within red blood cells, thus providing protection. However, sickle cell anemia does not eliminate the risk of clinical complications associated with anemia, which can be life-threatening.
5 CONCLUSIONS:
In the present study, it was observed that a significant number of children in tropical and subtropical regions carry sickle cell hemoglobin (HbS), providing evolutionary protection against malaria by reducing parasite levels within endothelial cells. This protective mechanism involves intravascular sickling and premature destruction of parasitized cells.
Despite the protective effect against malaria, children with HbS face mortality risks due to potential clinical complications associated with severe anemia, which can be life-threatening. Given these observations, it is imperative that endemic regions receive free antimalarial prophylaxis and effective treatment for sickle cell anemia. Implementing comprehensive plans aimed at reducing malaria prevalence and mortality rates across populations, regardless of sickle cell status, is essential.
6 REFERENCES
1. AMBROSE, E.E. et al. Surveillance for sickle cell disease, Bull World Health Organ. United Republic of Tanzania. 2020;98(12):859–68. Doi: https://doi.org/10.2471%2FBLT.20.253583.
2. CHIMUCO, K.S.M. Sickle cell disease as a protective factor against Malaria. Angolan Journal of Health Sciences, Hospital Municipal Olga Chaves, Lubango, Huila, Angola. 2022.
3. CONRAN, N. et.al. “Sickle Cell Vaso-Occlusion: The Dialectic between RedCells and White Cells”. Experimental Biology and Medicine, vol.246, no. 12, 1Apr.2021, pp. 1458–1472.
4. DEME/LY, I. et al. Malaria Characteristics in Children with Sickle Cell Disease.Open Journal of Pediatrics, v. 12, n. 1, p. 125–130, 6 jan. 2022.
5. ELENDU, C. et.al. “Understanding Sickle Cell Disease: Causes, Symptoms, andTreatment Options”. Medicine, vol. 102, nº 38, 2023.
6. ELEONORE, N.L.E., CUMBER, S.N, et al. Malaria in patients with sickle cellanaemia: burden, risk factors, and outcome at the Laquintinie hospital, Cameroon.BMC Infect Dis, 20, 40 (2020).
7. ERIDANI, S. Sickle cell protection from malaria. Hematology Reports, v. 3, n. 3,p. e24, 2011.
8.GERSHMAN, M. et al. Yellow fever vaccine and malaria prevention information, by country. Centers for Disease Control and Prevention (CDC). USA, 2024. Availablet:https://wwwnc.cdc.gov/travel/yellowbook/2024/preparing/yellow-fever-vaccine-maria-prevention-by-country.
9.GONG, L. Biochemical and immunological mechanisms by which sickle cell trait protects against malaria. Malar J., 2013;12(317):1–9.
10. MANGLA, A. et.al. Sickle Cell Anemia. StatPearls Publushing, 2022.
11. MARIE, C. et al. Malaria. Global Medical Knowledge, 2022. Available at: ttps://www.msdmanuals.com/pt-br/profissional/doen%C3%A7as-infecciosas/protoo%C3%A1rios-extraintestinais/mal%C3%A1ria.
12. MINISTRY OF HEALTH. Malaria Treatment Guide in Brazil. Secretaria de Vigilância em Saúde. Departamento de Imunização e Doenças Transmissíveis. Brasília: Ministério da Saúde, 2020. 76 p. : il. Access mode: World Wide Web: ISBN 978-85-334-2754-9.
13. NHOU, C.M. et al. Influence of the sickle cell trait on Plasmodium falciparum infectivity from naturally infected gametocyte carriers. BMC Infect Dis, 23, 317 (2023). https://doi.org/10.1186/s12879-023-08134-x.
14. OPPONG, M. et al. Prevalence of sickle cell disorders and malaria infection in children aged 1–12 years in the Volta Region, Ghana: a community-based study. Malaria Journal, v. 19, n. 1, 23 nov. 2020.
15. REAL, E. et al. “A Single-Cell Atlas of Plasmodium Falciparum Transmission through the Mosquito.” Nature Communications, vol. 12, no. 1, 27 May 2021, p.3196.16. SATO, S. “Plasmodium—a Brief Introduction to the Parasites Causing Human Malaria and Their Basic Biology.” Journal of Physiological Anthropology, vol. 40, no. 1, 7 Jan. 2021.
17. WORLD HEALTH ORGANIZATION. World Malaria Report 2022. World Health Organization, 2022.
18. UYOGA, S. et al. Sickle cell anemia and severe Plasmodium falciparum malaria: a secondary analysis of the Transfusion and Treatment of African Children Trial (TRACT). Lancet Child Adolesc Health; 6(9): 606-613, 2022. Mdl-35785794.
¹ Discente em enfermagem pela Universidade Maurício de Nassau (UNINASSAU)
² Farmacêutica pela Universidade Maurício de Nassau ( UNINASSAU)
³ Departamento de enfermagem pelo Centro Universitário Brasileiro (UNIBRA)
⁴ Enfermeiro pela Universidade Federal de Pernambuco (UFPE)
⁴ Mestre em Ciências da Saúde Universidade Federal de Pernambuco (UFPE)
