BIOÉTICA NA PRODUÇÃO TECNOLÓGICA DA UNIVERSIDADE FEDERAL DE SERGIPE
REGISTRO DOI: 10.5281/zenodo.11372814
Tiago de Melo Ramos
Ana Carla Barbosa Canuto
Helder Tulio Rodrigues de Souza
Robson Roberto Souto Santos
Thayslane de Melo Costa
Robélius De-Bortoli
Abstrat
Currently, ethics committees are responsible for analyzing and evaluating research projects developed in the country, seeking to ensure respect for the ethical principles of research and the rights of human beings and animals. Patents are indicative of production and technological innovation in the country, being a source of interesting data to identify products and processes developed by inventors, producers and creators in addition to inventors. The study aimed to analyze patent productions that were filed by inventors and/or researchers from the Federal University of Sergipe at the National Institute of Intellectual Property, and which had approval from the ethics committee. In its methodology, exploratory and explanatory research was chosen using the systematic review analysis technique to collect information. In which, through patentometrics, 278 patent applications were analyzed. It was possible to verify that patent applications that dealt with human beings or animals, mainly those belonging to classification B and C, had the analytical and evaluative treatment of the Research Ethics Committee, showing commitment and responsibility with ethics in research.
Keywords: Intellectual property; Human rights; Technology
The present study was carried out with the support of the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES)
INTRODUCTION
The process of evolution of humanity is made up of a series of factors, ruptures, milestones and mainly by man’s constant desire for evolution. Advanced studies on the human species, the emergence of new methods and techniques to combat ills and illnesses in society at each time, pushed human beings to delve deeper into the basic concepts of life. Instigated by desires and desires to discover cures, create medicines, develop foods that resist pests and soil peculiarities, society advanced and presented many solutions to various problems (Brochado, 2023). However, it is necessary to reflect on the risks and ‘limits’ of advances. It is from the perspective of this concern that bioethics emerges. Therefore, there was a concern with respect for the ethical principles of life, a respectful look at each living being, the systematization of rules and boundaries for the means to find solutions (Lolas Stepke, 2010).
Through the study by Dall’Agnol (2022), it is possible to conceptualize and understand bioethics as a branch of science that has human life and its relationships with innovations and technologies as its object of study. Having as a structuring principle to encourage the discussion of ethical problems that are linked to life, ensuring that man’s intervention does not exceed the ethical limits on life. Bioethics is still based on the fundamental principles that are autonomy, taking care of the intrinsic value of people and the need to take moral decoctions, non-maleficence that addresses the care of medicine with human life, beneficence aiming to maximize problems and enhance the benefits to life and justice or equity that addresses fair treatment of life (Ferrari, 2017).
Through the principles of bioethics it was possible to establish limits and develop studies that aimed to discuss the implications of scientific methods on life, and one of the means developed were ethics committees and later institutionalized in Universities, today, regulated by CAPES and submitted to the platform Brazil. According to Neto and Franco (2019), ethics committees emerged around 1960 and 1970 in the United States with a multidisciplinary character and developed in close relation to bioethics, driven by human rights, thus having their dissemination in universities and institutionalized in them. in contemporary times.
Currently, ethics committees are responsible for analyzing and evaluating research projects developed in the country, seeking to ensure respect for the ethical principles of research and the rights of human beings and animals. The basic principles are the requirement that research carried out with human beings or animals must pass through the committee, demonstrating compliance with the criteria and rules established based on ethics (Martins, Junqueira and De Araújo, 2021).
Recognizing the work developed by the Research Ethics Committee and its commitment to bioethics, this study aims to analyze the production of patents that were deposited by inventors and/or researchers from the Federal University of Sergipe at the National Institute of Intellectual Property, and which were approved by the Research Ethics Committee.
METHOD
This is an exploratory and explanatory research using the systematic review analysis technique to collect information. In the same proportion as other literature analysis methods, systematic analysis aims to integrate information from a given object of study, enabling a compilation of specific information and evidence. It consists of three stages where the objective of the review is initially defined, then the literature to be analyzed or document is delimited and finalized with the selection of the material or sample that will be used (Roever, 2020).
The research was carried out through patent analysis using the patentometry technique, which is used to analyze patent banks in a more assertive way and prospect the technological and inventive universe. Patent applications are available in the database of the (INPI) National Institute of Intellectual Property in the year 2024, through the following electronic address https://busca.inpi.gov.br/pePI/jsp/patentes/PatenteSearchBasico.jsp . The search process was carried out through the CNPJ of the Federal University of Sergipe (13.031.547/0001-04). Initially, accessing the WEB Portal of the National Institute of Intellectual Property, clicking on the advanced search tab and then on the applicant, holder and inventor field, entering the UFS CNPJ, the results of 278 patent applications were obtained. To analyze the patents, the Free and Informed Consent Form or ICF was searched. The patent applications were stratified into those that present the TCLE and those that do not, inserted into graphs and figures and subsequently discussed.
RESULTS AND DISCUSSION
The data selected, analyzed and discussed here are patents that were requested at the National Institute of Intellectual Property (INPI), with the intention of discussing and reflecting on scientific production and its relationship with the ethics and research committee of the Federal University of Sergipe . Patents are significant indicators of innovation and research, and for this reason, we chose to analyze them.
Patents can be understood as a product or process demanded from the human intellect, in Brazil through law no. 9,279/96 they are classified as invention patent a (PI) and utility model patent a (UM) (De- Carli et al. 2021). Through a patent, an inventor can protect and guarantee that his rights are not violated by third parties, being requested through the National Institute of Intellectual Property, where applications are requested and patents are deposited.
For Amaral (2023), patents are indicative of production and technological innovation in the country, being a source of interesting data to identify products and processes developed by inventors, producers and creators. Therefore, through patents it is possible to map innovation and production. Here, the patents were used to identify which area of production served the CEP, the proposing institution, the Federal University of Sergipe UFS in the research and whether the research process was approved by the ethics and research committee.
The Research Ethics Committee (CEP) has the important task of applying the regulatory standards and guidelines for research involving human beings, prioritizing the interests of research participants, defending their integrity and dignity, aiming to cooperate in the research construction process and respecting the ethical standards (Barbosa, et al. 2020). Based on CNS Resolution No. 466/12, which defines that all research involving human beings will need to be evaluated by a research committee.
Therefore, it is shown in graph 1 which patent applications were submitted to the ethics and research committee, as well as their respective deposit areas at the INPI through the International Patent Classification or IPC. Therefore, it is possible to see in graph 1 that there is a greater concentration of patent production that passed through the ethics and research committee of the Federal University of Sergipe (UFS) section A61K 31/045 which generally corresponds to devices or methods specially adapted to transform pharmaceutical products into certain physical or administration forms, followed by categories A01N 65/08, A61K 36/185, A61K 36/53 and A01N 65/36 with records greater than or equal to 5 deposits. Right behind the A61K 31/05, A61K 36/47 and A61K 9/107 respectively in the section ….. And the others with 3 or less presentation on the chart.
It must be taken into account that all the patents in graph 1 are related to the treatment of animals and aimed at humans. In this way, demonstrating commitment to the standards and guidelines of the UFS ethics and research committee. The information shown in figure 1 is in line with the data presented in the study Ethics in research: a study on doctoral theses in education by Menezes (et al. 2020) which presented a low incidence of research in doctoral programs related to human beings with authorization from the ethics committee. However, the study presented theses that were produced in the area of education and, perhaps, this factor led to the discrepancy in relation to the data from this research, which contains patents related to CEP health sections.
Figure 1 – Frecuency of Internatinal Patent classifications.
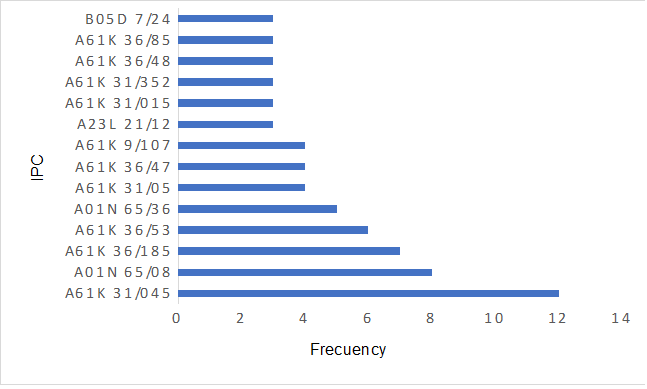
Analyzing the production of patents not only provides an indication of innovation at the Federal University of Sergipe UFS, but also points to the institution’s commitment to ethics in research. Because, federal universities are largely responsible for scientific and technological development in Brazil, as shown by data collected in the work of Ventura and Oliveira (2022), which found respect for the standards of the National Research Council, supporting the link between research and ethics.
In another study, developed by Heinz, 2021 with the aim of understanding the relationship between ethics and research carried out within their areas of knowledge, it was found that almost 60% of interviewees never used the ethics committee in their research, around 30 % almost never submitted and only 7.2% submitted. However, the majority consider the committee important, and attribute bureaucracy and excessive time as obstacles in the process. When compared with the data presented in graph 1, it appears that there has been a significant change in the process of submitting research to the ethics committee. The incident may be related to the efficiency of the ethics committee or the INPI’s requirements in the patent submission process.
Below, patent applications that did not receive approval from the ethics committee are highlighted, in addition to their IPC figure 2 is characterized by the IPC field and section field, in which it is possible to check the IPC of patents for which it was not possible to verify the approval of the ethics committee in their documents, and the sections in which these patents were categorized are also presented.
Figure 2 – International Patent classifications and Sections
IPC Section A23B 7/024, A23J 1/08, A23L 11/00, A23L 25/00, A23C 19/04, A23C 9/13, A23L 2/52, A23J 3/14, A23P 20/00, A23C 9/18, A23B 7/02, A23P 10/30, A23L 33/135, A23L 21/12, A23D 9/007, A23L 33/185, A23B 7/02, A23P 20/10, A23B 4/044, A23L 2/52, A23C 11/10, A23L 21/12, A23L 3/3472, A23L 21/20, A23L 21/20. Human and food needs; tobacco A61M 16/06, A61K 36/05, A61K 47/10, A61K 33/00, A61K 8/92, A61B 5/00, A61K 36/53, A61K 36/746, A61K 9/107, A61M 5/142, A61K 8/37, A61K 36/185, A61L 9/20, A61K 31/295, A61M 5/32, A61K 36/185, A61K 9/14, A61K 31/167, A61K 8/98, A61L 27/24, A61K 31/353, A61L 27/44, A61L 27/20, A61K 9/70, A61F 2/28, A61B 5/01, A61K 9/107, A61K 36/28, A61K 31/045, A61K 31/045, 36/185, A61K 31/045, A61K 36/47, A61K 36/85,A61K 36/85,A61K 31/122, A61K 8/24, A61K 31/045, A61B 6/14, A61K 47/40. Human health needs; life savings; fun G01N 21/59, G01N 15/08, G01N 21/01, G01N 33/14, G01N 33/15, G09B 23/28, G01B 11/30, G01T 1/02, G01N 23/04, G01V 9/00, G06Q 10/10, G01V 3/10, G01T 1/29, G01N 1/10, G01T 1/169. Physics: meters, tests; Computer calculations or assemblies C07K 14/44, C01B 32/182, C12P 1/00, C12C 12/00, C03C 3/14, C13K 1/06, C03C 3/076, C09B 61/00, C12P 19/14, C12N 1/22, C08H 8/00, C01B 32/198, C01B 32/198, C04B 35/44, C07C 39/06, C04B 18/14, C12P 1/02, C08L 23/08, C08B 37/16, C12P 21/00, C08H 1/00, C12N 11/10 C12P 19/06, C12N 11/12, C12P 15/00, C05G 3/04, C12N 11/12, C12M 1/107, C01B 32/324, C02F 1/58, C12P 1/04 C10B 53/02, C12P 5/02, C12P 7/62, C12P 7/62, C23C 30/00, C12P 1/02, C12N 9/50, C08K 3/30, C04B 35/622 C02F 1/28, C11D 7/42, C12N 15/10, C11D 7/42, C12P 5/02, C10L 5/42, C04B 41/86, C01B 31/12, C01B 33/14 C08G 69/48, C12F 3/10, C08L 5/00, C10L 5/42. Chemistry, metallurgy; organic chemistry, biochemistry, beer, spirits, wine, vinegar, microbiology, enzymology or genetic engineering B65D 81/26, B32B 23/04, B01D 53/04, B01D 53/02, B01J 20/24, B01J 2/28, B01J 20/30, B01J 23/52, B05B 5/08, B01D 39/02, B29C 45/00, B05D 7/24, B01J 19/24, B05D 7/22, B01D 11/02, B05D 7/24, B01J 20/22, B03B 1/04, B01J 39/00. Execution of operations; transport, packaging, storage, handling of fine or filamentary material E01C 23/01. Fixed constructions F24S 10/50, F25B 29/00, F03D 7/02. Mechanical Engineering; lighting; heating; weapons; explosion H01H 85/02 Electricity
The data obtained and presented in table 1 show that of the patent applications made through the UFS, only section D; textiles and paper do not make up the table. Sections A, B, C, E, F, G and H appear as requested requests and which, for some reason, do not have authorization from the ethics committee. In which, section A and section C stand out as major areas of concentration of technological production at UFS. Sections that are related Human needs (section A) and Chemistry; metallurgy (section B).
According to the ethics and research committee through Resolution No. 674, of May 6, 2022, which provides for the classification of research and the processing of research protocols in the CEP/Conep System, states that research must follow the rite ethics and research. In which it systematizes and organizes research according to the following logic:
Art. 4 Research involving human beings can be classified according to their procedure, divided into two types: I – Studies that involve intervention in the human body; II – Studies that do not involve intervention in the human body. Art. 5° The research procedure that involves intervention in the human body may or may not have an invasive nature in the physical dimension.
From this perspective, even if the research does not have direct contact with human beings, it must be submitted to the committee, because, as expressed in article 4 in item II, studies that do not involve intervention in the human body must be submitted and analyzed, further reinforcing in article 5 which emphasizes whether or not it has an invasive nature in the physical dimension.
Therefore, the patents presented in table 1, even if they do not involve direct contact with human beings, should be approved by the ethics committee. Explaining the reason for non-submission is not the purpose of this research, for this it would be necessary to carry out a new study seeking to find out the reason for non-submission.
It is also worth highlighting that according to resolution no. 674, of May 6, 2022, there is a classification of research in committees and depending on this classification, the process requires less time for analysis and opinion. Below is how searches can be classified.
“Chapter V RESEARCH TYPIFICATION Art. 6 Research is classified, according to the study design and procedure, into three types: A, B and C, as set out in Annex I of this Resolution. Ministry of Health / National Health Council. Pg. 14. 2022” In this organization we have type A, being the research category intended for those studies that aim to describe or understand phenomena that happened or happen in the daily life of the research participant and that require intervention in the human body.
In classification B we have studies that deal with intervention in the human body, whether or not there is a need for an invasive procedure, requiring a sub-typing for targeting. Type C includes studies that aim to verify the effect of a product or technique under investigation, deliberately applied to the participant as a result of the research, prospectively, with a control group or not.
Therefore, through typification it is understood that research that has an A, B or C classification must be submitted to the analysis process of the ethics and research committee. And the fact that the patents presented in figure 3 do not present invasive procedures involving human beings required analysis, seeking to ensure greater safety in the research process.
The International Patent Classification or IPC is a universal and systematized system for categorizing, organizing and archiving documents related to patents, using specific fields called sections. In this way, making it easier for interested parties to search and access information more efficiently (DA CUNHA, 2021).
Figure 3 – Sections of International Patent Classifications
Sections INTERNATIONAL PATENT CLASSIFICATION (IPC) A Human needs B Execution of operations; transport C Chemical; metallurgy D Textiles; paper E Fixed constructions F Mechanical Engineering; lighting; heating; weapons; explosion G Physical H Electricity
The IPC is mainly composed of a combination of letters and numbers, subdividing technological production into 8 sections or groups. Described in table 2, in a more specific way it is possible to interpret it as follows: A23L 21/20 in this specific registration we have the letter A representing the section of the patent, the number 23 the class in which it is characterized, the letter L the subclass , 21 the belonging group and 20 the Rezende subgroup (et al. 2023). Through this standard, patents are distributed and stored. Here, the IPC was used to identify in which sections the patents produced at the UFS were filed and subsequently understand which were submitted to the ethics committee and which did not pass. As discussed in table 1.
CONCLUSIONS
Through this research it was possible to verify that patent applications that dealt with human beings or animals, mainly those belonging to classification B and C, have the analytical and evaluative treatment of the Research Ethics Committee, showing commitment and responsibility with ethics in the search.
The largest area of concentration of patent applications filed is section A61K 31/045, which generally corresponds to devices or methods specially adapted to transform pharmaceutical products into certain physical or administration forms. A large portion of the requests that did not receive approval from the ethics committee are distributed in sections A, B, C, E, F, G and H, with a greater prevalence in sections A and C.
According to Resolution No. 674, of May 6, 2022, research whether or not dealing with human beings, directly or indirectly, must be submitted to the Research Ethics Committee, and must be typified and evaluated.
However, this research is aimed at researchers, teachers and inventors who want to reflect on the research and ethics scenario, providing an analysis of the production of innovation and its relationship with the ethics and research committee.
REFERENCES
AMARAL, Ricardo Maia do. Trâmite prioritário de patentes em processos pertencentes aos institutos federais do Nordeste: processo estratégico para desenvolvimento do sistema regional de inovação. Dissertação de Mestrado. 2023. Disponível em: https://repositorio.ifpb.edu.br/handle/177683/2851/. Acesso em: 02 jan. 2024.
BROCHADO, Mariah. Inteligencia Artificial e Ética: um diálogo com Lima Vaz. Kriterion: Revista de Filosofia, v. 64, p. 75-98, 2023. https://doi.org/10.1590/0100-512X2023n15404mb. Disponível em: https://www.scielo.br/j/kr/a/4NKGBGSPn3J8KDBb44VBTBf/. Acesso em 12 jan. 2024
DA CUNHA, Gilberto José. A prospecção tecnológica a partir de bases de dados de patentes. Revista Panorâmica online, v. 34, 2021. Recuperado de https://periodicoscientificos.ufmt.br/revistapanoramica/index.php/revistapanoramica/article/view/1426 Acesso em: 8 jan. 2024
DALL’AGNOL, Darlei. Bioética: princípios morais e aplicações. PUCPRess, 2022. Disponível em: https://books.google.com.br/books?hl=pt-BR&lr=&id=a1OZEAAAQBAJ&oi=fnd&pg=PT7&dq=.+Bio%C3%A9tica:+princ%C3%ADpios+morais+e+aplica%C3%A7%C3%B5es&ots=LcUqSDvKfd&sig=waXnLthyZy24DrwyEPRYIYfqncs&redir_esc=y#v=onepage&q=.%20Bio%C3%A9tica%3A%20princ%C3%ADpios%20morais%20e%20aplica%C3%A7%C3%B5es&f=false. Acesso em 05 jan. 2023
DE ALMEIDA, João Beccon e; FRANCO, Túlio Batista. Análise das publicações sobre os comitês de ética em pesquisa em Scientific Electronic Library Online (Scielo). Revista Latinoamericana de Bioética, v. 19, n. 1, p. 27-50, 2019. https://doi.org/10.18359/rlbi.3641. Disponível em: http://www.scielo.org.co/scielo.php?pid=S1657-47022019000100027&script=sci_arttext/. Acesso em 05 jan. 2024
DE MENEZES, Jones Baroni Ferreira; DA SILVA LIMA, Ana Michele; NUNES, João Batista Carvalho. Ética na pesquisa: um estudo sobre teses de doutoramento em educação. Horizontes, v. 38, n. 1, p. e020051-e020051, 2020. https://doi.org/10.24933/horizontes.v38i1.897. Disponível em: https://novoshorizontes.usf.emnuvens.com.br/horizontes/article/view/897. 16 jan. 2024
DE-CARLI, Eduardo; FREGA, Paula Andréa e, ALVES, Salvador José Roberto. Caracterização da produção de depósitos de patentes de universidades brasileiras. 2021. Disponível em https://repositorio.altecasociacion.org/handle/20.500.13048/1341. Acesso em 09 jan 2024.
FERRARI, Renata Rodrigues. Princípios Da Bioética. IN TOTUM-Periódico de Cadernos de Resumos e Anais da Faculdade Unida de Vitória, v. 4, n. 1, 2017. Disponível em: https://revista.fuv.edu.br/index.php/intotum/article/view/1504. Acesso em 12 jan. 2024
HEINZ, Gabriela; Zucatto, Luís Carlos; Quadros, Claudemir de; Troian, Alessandra. Comitê de ética em pesquisa com seres humanos da Universidade Federal de Santa Maria: uma investigação acerca de percepções de seus usuários. Repositório digital da UFSM. 2021.Disponível em: http://repositorio.ufsm.br/handle/1/24047. Acesso em 20 jan. 2024
LOLAS STEPKE, Fernando. Acta Bioethica: una década de historia. Acta bioethica, v. 16, n. 2, p. 115-118, 2010. http://dx.doi.org/10.4067/S1726-569X2010000200002. Disponível em: https://www.scielo.cl/scielo.php?pid=S1726-569X2010000200002&script=sci_arttext. Acesso em 15 jan 2024.
MARTINS, Valéria Farinazzo; JUNQUEIRA, Michelle Asato; DE ARAUJO, Renata Mendes. Ética da Pesquisa em Sistemas de Informação: Por que e como submeter meu projeto ao Comitê de Ética?. Sociedade Brasileira de Computação, 2021. Disponível em: https://sol.sbc.org.br/livros/index.php/sbc/catalog/view/64/282/531. Acesso em 16 jan. 2024
REZENDE, Nicolas; DALIP, Daniel Hasan; Brandão, Michele A; Vasconcelos, Marisa A. Elaboraçao de um Conjunto de Dados sobre o Registro de Patentes no Brasil. In: Anais do V Dataset Showcase Workshop. SBC, 2023. p. 99-108. https://doi.org/10.5753/dsw.2023.233875. Disponível em: https://sol.sbc.org.br/index.php/dsw/article/view/25509. Acesso em 26 jan. 2024
ROEVER, Leonardo. Guia prático de revisão sistemática e metanálise. 2020. Disponível em: https://books.google.com.br/books?hl=pt-BR&lr=&id=w0LWDwAAQBAJ&oi=fnd&pg=PT8&dq=Guia+pr%C3%A1tico+de+revis%C3%A3o+sistem%C3%A1tica+e+metan%C3%A1lise&ots=__RDmSGZfs&sig=NvNZC9T3DffXVRXI6CNuk9kMRd4&redir_esc=y#v=onepage&q=Guia%20pr%C3%A1tico%20de%20revis%C3%A3o%20sistem%C3%A1tica%20e%20metan%C3%A1lise&f=false. Acesso em 26 jan. 2024
SILVA, Rodrigo Barbosa e; CONCEIÇÃO, Vida Kamila Pinheiro da. O comitê de ética em pesquisa como espaço de formação continuada do professor universitário. Revista Internacional de Educação Superior, Campinas, SP, v. 6, p. e020033, 2019. DOI: 10.20396/riesup.v6i0.8656515. Disponível em: https://periodicos.sbu.unicamp.br/ojs/index.php/riesup/article/view/8656515/.Acesso em: 11 jan. 2024
SPEZIALI, Marcelo Gomes; NASCIMENTO, Raphael da Silva. Patentometria: uma ferramenta indispensável no estudo de desenvolvimento de tecnologias para a indústria química. Química Nova, v. 43, p. 1538-1548, 2021. https://doi.org/10.21577/0100-4042.20170620/. Disponível em: https://www.scielo.br/j/qn/a/Kk7D8sML8f8BY93X3RXXFHD/?lang=pt. Acesso em: 02 fev. 2024
VENTURA, Miriam; OLIVEIRA, Suelen Carlos de. Integridade e ética na pesquisa e na publicação científica. Cadernos de Saúde Pública, v. 38, p. e00283521, 2022. https://doi.org/10.1590/0102-311X00283521 Disponível em: https://www.scielosp.org/article/csp/2022.v38n1/e00283521/pt/. Acesso em 04 fev. 2024.
