AVALIAÇÃO DA RESPOSTA AO ESTRESSE OXIDATIVO EM CIRURGIA MINIMAMENTE INVASIVA E LAPAROTOMIA EM FELINAS APÓS OVÁRIO-HISTERECTOMIA
REGISTRO DOI: 10.5281/zenodo.12584628
Fabiane Azeredo Atallah1
André Lacerda de Abreu Oliveira2
Beatriz Pinheiro3
Carla Correia-Gomes4
Mayara Rodrigues Medina Gomez5
Leandro da Silva Gardel6
ABSTRACT
Objectives: The purpose of this study was to examine the alterations in lipid peroxidation and protein carbonylation, two reliable biomarkers associated with oxidative damage, after laparoscopic or traditional ovariohysterectomy (OVH) in female cats. Methods: The study included forty healthy female cats, from which similar amounts of blood samples were collected under anesthesia before surgery, immediately after and 24 hours after surgery.Serum oxidative stress (OS) was evaluated by assessing protein carbonyl groups and 4-hydroxynonenal plasma levels, using immunoblotting techniques. Results: A loss of control over reactive oxygen species (ROS) production occurred in female cats after OVH, which lead to increased oxidative damage, particularly in the late post-operative period, suggesting that, traditional OVH is associated with an immediate increase of OS, while OVH by laparotomy is related with the risk of OS in the late period after surgery. Conclusion: Given that OS contributes to the pathogenesis of various diseases, OVH female cats may present an increased risk of OS-associated disorders. Relevance: OS is an integral part of the surgical stress response. Minimally invasive surgery causes fewer traumas, and thus attenuated stress response is anticipated. However, the occurrence of pneumoperitoneum is involved in the production of free radicals. A strategy to counteract the deleterious effects of OS after OVH may be advisable, although further studies are necessary before a definitive recommendation. Key words: feline, reactive oxygen species, surgical manipulation, intestine, laparoscopic, lipid peroxidation, protein carbonyl.
RESUMO
Objetivos: O objetivo deste estudo foi examinar as alterações na peroxidação lipídica e na carbonilação de proteínas, dois biomarcadores confiáveis associados ao dano oxidativo, após ovariohisterectomia laparoscópica ou tradicional (OVH) em gatas. Métodos: O estudo incluiu quarenta gatas saudáveis, das quais quantidades semelhantes de amostras de sangue foram coletadas sob anestesia antes da cirurgia, imediatamente após e 24 horas após a cirurgia. O estresse oxidativo (OS) sérico foi avaliado pela avaliação dos grupos carbonila da proteína e dos níveis plasmáticos de 4-hidroxinonenal, usando técnicas de immunoblotting. Resultados: Ocorreu uma perda de controle sobre a produção de espécies reativas de oxigênio (ROS) em gatas após OVH, o que levou a um aumento do dano oxidativo, particularmente no pós-operatório tardio, sugerindo que o OVH tradicional está associado a um aumento imediato de OS , enquanto a OVH por laparotomia está relacionada com o risco de OS no período tardio após a cirurgia. Conclusão: Dado que a OS contribui para a patogénese de várias doenças, as gatas OVH podem apresentar um risco aumentado de doenças associadas à OS. Relevância: OS é parte integrante da resposta ao estresse cirúrgico. A cirurgia minimamente invasiva causa menos traumas e, portanto, uma resposta atenuada ao estresse é antecipada. No entanto, a ocorrência de pneumoperitônio está envolvida na produção de radicais livres. Uma estratégia para neutralizar os efeitos deletérios do OS após OVH pode ser aconselhável, embora mais estudos sejam necessários antes de uma recomendação definitiva. Palavras-chave: felino, espécies reativas de oxigênio, manipulação cirúrgica, intestino, laparoscopia, peroxidação lipídica, proteína carbonilada.
INTRODUCTION
Laparoscopy is widely used because it offers many advantages to patients including reduced postoperative pain, shorter hospital stay (shortened convalescence) (YIANNAKOPOULOU et al., 2013), reduction of intraoperative bleeding, fewer blood product transfusions and shorter care in the post anesthesia unit (KUMARI et al., 2022; DUPRÉ et al., 2009). Laparoscopic surgery through smaller abdominal incisions and less tissue manipulation causes fewer traumas and thus is expected be followed by a less pronounced stress response (YIANNAKOPOULOU et al., 2013; KUMARI et al., 2022). However, this procedure requires the completion of a pneumoperitoneum for adequate visualization of the operative manipulation (SAMMOUR, T. et al., 2009). Depending on the pressure level of the gas used, this technique may cause severe inflammatory and metabolic responses, which induce hemodynamic alterations in patients (WIESENTHAL, J. D. et al., 2011; UMANO, G. R. et al., 2021), can affect several homeostatic systems leading to changes in acid-base balance, blood gases, cardiovascular systems and respiratory physiology (KEEGAN, R. F.; WEBB, C. B., 2010). The insufflation and deflation of the pneumoperitoneum is like a model of ischemia-reperfusion injury, and thus the induction of oxidative stress and OS response is anticipated (SAMMOUR, T. et al., 2009).
Numerous studies reported an increase of OS in both women and female laboratory animals after ovariectomy (KEEGAN, R. F.; WEBB, C. B., 2010; TECLES, F. et al., 2015). A higher risk of many diseases associated with OS, such as cardiovascular diseases, renal diseases, osteoporosis and Parkinsonsm, has been observed in women after ovariectomy (TECLES, F. et al., 2015). In cats there have been reports of renal and liver disease, as well diabetes (KEEGAN, R. F.; WEBB, C. B., 2010). Even so the impact of minimally invasive surgery on OS is not fully understood and the data are not ideal and are often controversial (TECLES, F. et al., 2015). In fact, there is little information about the evaluation of antioxidative/oxidative status in ovariohysterectomized cats.
MATERIALS E METHODS
Experimental design
This study includes 40 healthy domestic cats, ASA 1, after performing tests, ranging in age from 6 months to 3 years that were referred to the Veterinary Medical Teaching Hospital of University of Porto for elective ovariohysterectomy. Cats were randomly selected to four groups, one for ovariohysterectomy (OVH) by laparotomy (n=10) and another for OVH laparoscopic group (n=10), anesthetized group for 13 minutes (n=10) and another for 43 minutes (n=10). None of the cats in either group were purebred. The study was performed according to the national guidelines after approval by the National Ethical Committee for Laboratory Animals (2007-07-27; document no. 018 939) and conducted in accordance with international standards on animal welfare as defined by the European Communities Council Directive of 2 November 1986 (86/609/EEC).
Sample collection
For each group, 1 mL of blood in three different times. The first collection was made immediately pre anesthesia (T1); the second was made immediately after the end of surgery (T2); and a third was made 24 hours after surgery (T3). The laparoscopic surgery took on average 43 minutes to perform while the laparotomy took on average 13 minutes. Blood samples were immediately frozen and kept at -80ºC until use.
Lipid peroxidation levels
The 4-hydroxy-2-nonenal (4-hydroxynonenal; 4-HNE) is a highly reactive aldehyde generated by the exposure of polyunsaturated fatty acids to peroxides and ROS. 4-HNE levels were quantified using a specific goat anti-4-hydroxynonenal polyclonnal antibody according to the method described by KRUMAN, I. et al. (1997) and collaborators.
Protein oxidation levels
Protein oxidation levels were evaluated by assessing protein carbonyl content in the samples, which is commonly used as a marker for OS (SPICKETT, C. M., 2013). Protein concentration of samples was then determined by using the BCA Protein Assay Kit (Pierce, Thermo Fisher) following the manufacturer’s instructions. Lysed samples were derivatized using 2,4-dinitrophenylhydrazine (DNPH) according to the method described by DIAS, T. R. et al. (2014) and collaborators.
Statistical analysis
The variables were tested to see if they followed a normal distribution using the KolmogorovSmirnov test with Lilliefors correction. To test if there were differences within times per group the Friedman test was used. The Wilcoxon signed-rank test was used to test the differences between pairs of times (before versus immediately after, before versus 24h, immediately after versus 24h). To test for differences between groups (laparotomy versus laparoscopy) the Mann-Whitney U test was used. This test was also used to test if there were differences between the animals that undergone surgery (laparotomy and laparoscopy) and the ones that were only under the effect of the anesthesia (control groups).
RESULTS AND DISCUSSION
The effect in ROS due to the surgery can be observed when we compare the animals that undergone surgery with the ones that suffered only the effect of the anesthesia (Figure 1A). The animals that were only anesthetized had a higher variation of lipid peroxidation at T2 when compared to the ones that suffer surgery, while the opposite was observed for T3 (Table 1). These differences were statistically significant (p<0.05) for each time period between the control groups and the surgery groups.
Within group comparison for the laparoscopy group showed statistically significant differences (p<0.0001) between times for protein carbonylation. The same was observed for lipid peroxidation (p<0.0001). For the laparotomy group no statistically differences were observed between times for protein carbonylation (p=0.058) and lipid peroxidation (p=0.209).
When comparing groups (laparotomy versus laparoscopy) there was a statistically significant difference between groups for T3 (24h after surgery) for the lipid peroxidation (p=0.038). The group that undergone laparotomy had higher values of lipid peroxidation compared to the group that undergone laparoscopy (Figure 1B).
The degree of OS was evaluated by measuring the final products of the generated injury using different biomarkers (DIAS, T. R. et al., 2014). The OS can be measured by the quantity of the ROS, the damage caused by ROS or by the levels of antioxidant response generated (KRUMAN, I. et al., 1997; SPICKETT, C. M., 2013).
Previous studies have shown that there is not a single biomarker that can really represent OS and the result of each can vary immensely (DIAS, T. R. et al., 2014) , taking this into account in the present study we used two different biomarkers: The 4-HNE chosen as a measure of lipid peroxidation due to form a highly stable adduct with protein side specific chains (KRUMAN, I. et al., 1997; SPICKETT, C. M., 2013) and protein oxidation levels assessed by the protein carbonyl content since this is an extremely reliable and stable biomarker.
On the comparison between the two surgery groups and the groups that were only anesthetized, there was a statistically significant difference. There was a tendency to normalization of the biomarkers values in T3 at the anesthetized groups, suggesting that the results obtained in this study are due to surgery and not only the anesthesia.
Each biomarker had different results. Comparing the laparoscopy and laparotomy groups there was significant difference for Lipid Peroxidation in T3 were laparotomy group had higher values. The possible reason for such an event is found in the fact that the plasma membrane is one of the most affected by ROS damage, inducing changes in the structure and permeability of cell membranes (POERWOSUSANTA, H. et al., 2020, 2022; SAMPURNO, S. et al., 2019).
These results are concordant with studies that suggests a lower OS by the laparoscopic technique in wich pneumoperitoneum triggers ischemic and reperfusion syndrome, even representing a minimally invasive technique. It is believed that these findings may be related to the use of a moderate pneumoperitoneum pressure (UMANO, G. R. et al., 2021). That would explain how the laparoscopy group having longer surgical time (average 43 minutes), with a 10 mmHg pressure did not provide during this period higher OS damage when compared with laparotomy group. Suggesting that the laparoscopic procedure did not become an ischemia reperfusion model as cited by several authors (UMANO, G. R. et al., 2021).
SAMMOUR, T. et al. (2009), in a retrospective study for laparoscopy in relation with OS, demonstrated that time and the intra-abdominal pressure during pneumoperitoneum, can significantly interfere with the hemodynamic changes and production of OS.
According to POERWOSUSANTA et al. (2020), Sprague-Dawley rats submitted to 60-min laparoscopy with carbon dioxide (CO2) pneumoperitoneum of 8, 10 and 12 mmHg had a progressive and significant increase in the oxidative stress index (OSI), in the infiltration and peritoneal degranulation of mast cells, as well as a reduction in the thickness of the mesothelium in the peritoneum and small intestine.
In an experimental study with 30 Wistar rats, a group was submitted to pneumoperitoneum for 30 and another for 60 minutes, both with a 10 mmHg pressure. The first group showed no hemodynamic changes, which no longer occurred with the second (WIESENTHAL, J. D. et al., 2011). In that study the time which the animal was subjected to pneumoperitoneum seems to be relevant, which was not observed in the present study, since despite the time of surgical laparoscopy group was higher, the Lipid Peroxidation remained lower than in the group laparotomy in T3.
The protein carbonyl increase related to the laparotomy group matches with the report described by ANUP et al. (1999), which concluded through an experiment with mice that smooth handling of the intestine is able to induce OS. During the experiment, the animals underwent laparotomy and as a result suffered light manipulation in every segment of the small intestine (ID) for one minute without causing actual harm to the ID, only with the intestinal manipulation that occurs during abdominal surgery. This study indicated that the ID can be highly susceptible to injury during abdominal surgery and gentle handling of the intestines is able to induce OS.
SIMMY, T. et al. (2004) reported that every abdominal surgery involves intestinal manipulation to reach the organs below. They further argued that during surgery there might be a decrease in blood flow in the intestine due to a decrease of blood pressure triggering an ischemia and reperfusion injury as in the pneumoperitoneum. Despite the short time of surgery in the laparotomy group, the higher production of ROS may be justified by a growing body of experimental data claiming that the gastrointestinal tract is extremely sensitive to surgical stress, even in remote locations. It justifies the increased OS in the laparotomy compared with the stress caused by inflation of the laparoscopy.
However, the same study cited above noted that the greatest surgical stress damage was 1 hour after surgery and changes recovered within 24 hours after surgery. They concluded that it is likely that, in large abdominal surgery, the recuperation time can be much higher for the changes to reverse and sometimes they could be irreversible.
Into the present study, the greatest response of OS was at lipid peroxidation in 24 hours after surgery for the laparotomy group and when compared with the laparoscopy group there were statistically significant differences between groups. Other significant differences were observed within the laparoscopy group when comparing T2 and T3 time. The statistical significance may be due both to a reduction in the ability of antioxidant defenses to clean them in the related postoperative with its redistribution and increased consumption, as well as a delayed release of ROS (KEEGAN, R. F.; WEBB, C. B., 2010; KRUMAN, I. et al., 1997; SPICKETT, C. M., 2013).
According to KEHLET, H. (1999) and several authors, the debate on laparoscopic surgery decreasing the response to surgical stress is on an upward trend studies. We all know that any form of trauma, including surgery, results in OS, and that both laparoscopic and open surgical procedures can produce changes in several endocrine metabolic responses and changes in immune function, being more pronounced during open surgery.
CONCLUSION
In conclusion, our study showed that both laparotomy and laparoscopy caused an increase on OS. However, laparoscopic caused significantly less OS than laparotomy. Our findings suggest that laparotomy OVH is related with the risk of OS in the late period after surgery. Given that OS contributes to the pathogenesis of several chronic diseases, this may suggest an increased risk of disorders in ovariohysterectomized female cats; however, long-term future studies are required to confirm this hypothesis.
ACKNOWLEDGMENTS
The authors thank Dr. Marcus Falcão Clinical Director of the Veterinary Hospital of Bicuda and all his staff for the precious participation in this work.
FUNDING
This work was supported by the Coordination for the Improvement of Higher Level Personnel CAPES [€1000] and Foundation for State Research in Rio de Janeiro FAPERJ [$727 USD].
DECLARATION OF CONFLICT OF INTEREST
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally for the conception and writing of the manuscript. All authors critically revised the manuscript and approved of the final version.
REFERENCES
YIANNAKOPOULOU, E. et al. Minimally invasive surgery and oxidative stress response: What have we learned from animal studies? Surgical Laparoscopy, Endoscopy and Percutaneous Techniques, v.23, n.1, feb. 2013. Available from: <https://doi.org/10.1097/SLE.0b013e318278cf5f>. Accessed: Jul. 15, 2014. doi: 10.1097/SLE.0b013e318278cf5f.
KUMARI, A. et al. Oxidative stress and antioxidant activity in female dogs undergoing laparoscopic and open elective ovariectomy. Indian Journal of Animal Sciences, v.92, n.7, jul. 2022. Available from: <https://doi.org/10.56093/ijans.v92i7.102089>. Accessed: jan. 18, 2023. doi: 10.56093/ijans.v92i7.102089.
DUPRÉ, G. et al. Laparoscopic ovariectomy in dogs: comparison between single portal and two-portal access. Veterinary surgery: VS, v.38, n.7, p.818–824, oct. 2009. Available from: <https://doi.org/10.1111/j.1532-950X.2009.00601.x>. Accessed: jan. 18, 2023. doi: 10.1111/j.1532-950X.2009.00601.x.
WIESENTHAL, J. D. et al. Effect of Pneumoperitoneum on Renal Tissue Oxygenation and Blood Flow in a Rat Model. Urology, v.77, n.6, p.1508-15, jun. 2011. Available from:<https://doi.org/10.1016/j.urology.2011.02.022>. Accessed: jul. 11, 2014. doi: 10.1016/j.urology.2011.02.022.
UMANO, G. R. et al. The “Dark Side” of Pneumoperitoneum and Laparoscopy. Minimally Invasive Surgery, v.2021, p.1-9, may. 2021. Available from: https://doi.org/10.1155/2021/5564745>. Accessed: jan. 23, 2023. doi:10.1155/2021/5564745.
KEEGAN, R. F.; WEBB, C. B. Oxidative Stress and Neutrophil Function in Cats with Chronic Renal Failure. Journal of Veterinary Internal Medicine, v. 24, n.3, p.514–519, may-jun. 2010. Available from: <https://doi.org/10.1111/j.1939-1676.2010.0498.x>.Accessed: jul. 11, 2014. doi: 10.1111/j.1939-1676.2010.0498.x.
TECLES, F. et al. Serum biomarkers of oxidative stress in cats with feline infectious peritonitis. Research in Veterinary Science, v.100, p.12-17, jun. 2015. Available from:<https://doi.org/10.1016/j.rvsc.2015.02.007>. Accessed: apr. 14, 2016. doi:10.1016/j.rvsc.2015.02.007.
DIAS, T. R. et al. White Tea as a Promising Antioxidant Medium Additive for Sperm Storage at Room Temperature: A Comparative Study with Green Tea. Journal of Agricultural and Food Chemistry, v.62, n.3, p.608-617, jan. 2014. Available from:<https://doi.org/10.1021/jf4049462>. Accessed: apr. 14, 2016. doi: 10.1021/jf4049462.
KRUMAN, I. et al. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. The Journal of Neuroscience, v.17, n.13, p.5089-5100, jul. 1997.
Available from: <https://doi.org/10.1523/JNEUROSCI.17-13-05089.1997>. Accessed: apr. 14, 2016. doi: 10.1523/JNEUROSCI.17-13-05089.1997.
POERWOSUSANTA, H. et al. The effect of laparoscopy on mast cell degranulation and mesothelium thickness in rats. BMC Surgery, v.20, n.111, p.1-10, may 2020. Available from:<https://doi.org/10.1186/s12893-020-00775-y>. Accessed: jan. 18, 2023. doi: 0.1186/s12893-020-00775-y.
POERWOSUSANTA, H. et al. Dayak Onions (Eleutherine americana L Merr) Reduced Mesothelial Cell Detachment After Laparoscopy in Rats. Open Access Macedonian Journal of Medical Sciences, v.10(A), p.1321-1329, jan. 2022. Available from:<https://doi.org/10.3889/oamjms.2022.8297>. Accessed: jan. 18, 2023. doi: 10.3889/oamjms.2022.8297.
SAMPURNO, S. et al. Modes of carbon dioxide delivery during laparoscopy generate distinct differences in peritoneal damage and hypoxia in a porcine model. Surgical Endoscopy, v.10(A), p.1321-1329, oct. 2019. Available from: <https://doi.org/10.1007/s00464-019-07213-y>. Accessed: jan. 18, 2023. doi: 10.1007/s00464-019-07213-y.
SPICKETT, C. M. The lipid peroxidation product4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biology, v.1, n.1, p.145–152, jan. 2013. Available from:<https://doi.org/10.1016/j.redox.2013.01.007>. Accessed: apr. 15, 2016. doi:10.1016/j.redox.2013.01.007.
SAMMOUR, T. et al. Systematic review of oxidative stress associated with pneumoperitoneum. The British Journal of Surgery, v.96, n.8, p.836-50, aug. 2009. Available from: <https://doi.org/10.1002/bjs.6651>. Accessed: apr. 15, 2016. doi: 10.1002/bjs.6651.
ANUP, R. et al. Surgical stress and the small intestine: Role of oxygen free radicals. Annals Surgery, v.125, n.5, p.560–569, may 1999. Accessed: apr. 15, 2016. PMID: 10330946.
SIMMY, T. et al. Surgical Manipulation of the Intestine Results in Quantitative and Qualitative Alterations in Luminal Escherichia coli. Annals of Surgery, v.240, n.2, p.248-254, aug. 2004. Available from: <https://doi.org/10.1097/01.sla.0000133119.38175.97>. Accessed: apr. 15, 2016. doi: 10.1097/01.sla.0000133119.38175.97.
KEHLET, H. Surgical stress response: does endoscopic surgery confer an advantage?. World Journal of Surgery, v.23, n.8, p.801–807, aug. 1999. Available from:<https://doi.org/10.1007/s002689900583>. Accessed: apr. 15, 2016. doi:10.1007/s002689900583.
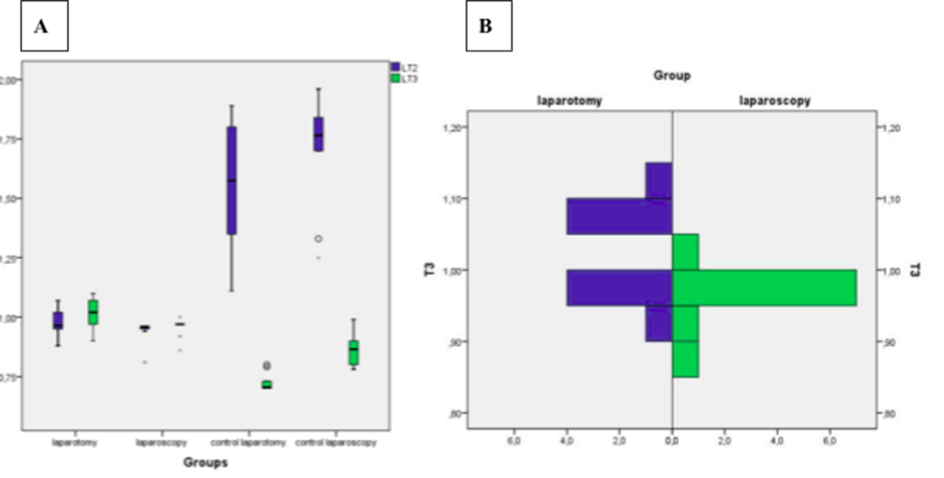
Figure 1 – (A) Boxplot of the lipid peroxidation results per group for the two time periods (T2 – immediately after surgery, T3 – 24h after surgery) and the four groups; (B) Histogram of the results of lipid peroxidation for time T3 (after 24h of the surgery) for the laparotomy and laparoscopy groups.
Table 1 – Descriptive results of the protein carbonyl and the lipid peroxidation for each group.
ROS Group Time Descriptive of the results Minimum Median Mean Maximum Protein carbonyl Laparotom y T2 0,97 1,03 1,13 1,54 T3 0,91 1,19 1,34 1,98 Laparosco py T2 1,02 1,02 1,04 1,14 T3 1,27 1,27 1,33 1,64 Lipid peroxidati on Laparotom y T2 0,88 0,97 0,98 1,07 T3 0,90 1,02 1,02 1,10 Laparosco py T2 0,81 0,96 0,94 0,96 T3 0,86 0,97 0,96 1,00 Control for laparotom y T2 1,11 1,58 1,55 1,89 T3 0,70 0,71 0,73 0,80
1 PhD em cirurgia. Médica Veterinária Autônoma. E-mail: fabianevet@hotmail.com.
2 PhD. Professor Associado de Técnica Cirúrgica da UENF. CEP 28013602, Avenida Alberto Lamego, n.2000, Campos dos Goytacazes, RJ, Brasil.
3Laboratory of Cytogenetics, ICBAS- Abel Salazar Institute for Biomedical Sciences, University of Porto, Portugal.
4Veterinary Epidemiologist. Epidemiology Research Unit, Future Farming Systems, Scotland’s Rural College (SRUC), Aberdeen, UK.
5 Aluna de graduação na Universidade de São Paulo (USP) em Medicina Veterinária. São Paulo, SP, Brasil.
6DVM, PhD. Assistant Professor of Surgical and Anesthesiology Department of Clinic Veterinary ICBAS-University of Porto, Porto, Portugal.
