REGISTRO DOI: 10.69849/revistaft/ra10202408222301
Meillyne Alves dos Reis1, Laís Bárbara Ferreira2, Ana Flávia de Carvalho Lima Biella3, Ana Claudia Souza Pereira4, Marcos André de Matos5
ABSTRACT
Introduction: Sexually transmitted infections (STIs) are a serious global public health problem, given the impact on the sexual and reproductive health of infected individuals and their partners. Therefore, this systematic review and meta-analysis is aimed to describe the vulnerability of police officers to STIs, as well as the associated factors.
Methods and analysis: Two independent researchers will carry out selection of studies in the databases Banco de Dados em Enfermagem (BDENF), Latin American and Caribbean Literature in Health Sciences (LILACS), National Library of Medicine – National Institutes of Health (NIH), Scientific Electronic Library Online (SciELO), SciVerse Scopus, and Web of Science, via Coordination for the Improvement of Higher Education Personnel (CAPES) by accessing the Federated Academic Community (CAFe), as well as extracting data and assessing the quality of studies. Data will be synthesized by the model of fixed effects or random effects, according to a heterogeneity test. Identification of the vulnerability of police officers to STIs, based on prevalence and incidence compared to the general population, will be assessed as a primary outcome. Variables associated with vulnerability will be evaluated as secondary results. The selection of articles will be carried out using the Rayyan software. Quantitative synthesis will be performed if the studies are homogeneous and provide results for meta-analysis. Otherwise, they will be synthesized using the narrative approach. Both the Begg and Mazumdar test and the Egger test will be used to assess small effects of the study. if p ≥ 0.05, the study will be considered free from publication bias.
Ethics and dissemination: The results of this systematic review and meta-analysis will be published in a peer-reviewed, high-impact journal. Ethical approval by a research ethics committee is not required because the data used are in the public domain.
Trial registration number: International Prospective Register of Systematic Reviews (PROSPERO) CRD42023422458.
Strengths and limitations of this study
► This protocol for systematic review and meta-analysis follows the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.
► Two independent reviewers will perform the study selection, data extraction, and assessment of the risk of bias.
► This study aims to provide a description of police officers’ vulnerabilities to sexually transmitted infections, as well as associated factors.
► Given that this study did not receive any financial support, it is limited to open access articles.
► Another limitation is the scarcity of publications dedicated to the theme addressed, demanding new studies and innovative health care proposals in the context of public safety.
INTRODUCTION
Sexually transmitted infections (STIs) represent a serious global public health problem, given the impact on the sexual and reproductive health of infected individuals and their partners. It is estimated that more than 1 million STIs are acquired daily worldwide, mainly affecting people between 15 and 49 years old, and the vast majority is asymptomatic.1
Since many STIs are asymptomatic or cause nonspecific symptoms, most often they have a late diagnosis. Consequently, they may spread without control and even be transmitted involuntarily. This phenomenon potentiates stigmatization, infertility, cancer, and pregnancy complications, and may also significantly increase the risk of being infected with the human immunodeficiency virus (HIV).2
Considering the magnitude of STIs/HIV/AIDS, the international community, through one of the Sustainable Development Goals, declared its commitment to ending the HIV/AIDS epidemic by 2030.3 However, the COVID-19 pandemic made the achievement of this goal impossible, due to inadequate coverage of health services, limited access to therapy, interruption of specialized services, discrimination and stigma, and also because of lack of awareness about adherence to condoms.4
Despite countless efforts to identify and implement interventions that can reduce risky attitudes, behaviors, and practices, changes in the sexual behavior still remain a complex challenge that has required numerous joint efforts from managers and scientists globally.5 6 This is more evident in vulnerable population groups that have little visibility in educational projects.7 8
Police officers are a category of civil servants whose activities are unique when it comes to risk issues, as they play a structuring role in working, environmental, and relational conditions.9 10 Studies have indicated that police officers have vulnerabilities such as the use of dating apps with multiple casual relationships,7 8 homosexual relationships,11 sexual relationships without condoms,12 alcohol use,10 multiple sexual partnerships,9 difficulty accessing health services, lack of knowledge about STIs, and a sense of invulnerability.13 14 15
These data are alarming, since simple and effective prevention, screening, diagnosis, and treatment are available in a large number of countries. Furthermore, this trend shows that longstanding factors such as lack of access to regular medical care, discrimination, and stigma continue to impede quality sexual health for all who need it.
Abundant evidence has demonstrated the increased prevalence of STIs and the need for strategic and timely interventions for police officers.9 14 In order to create and implement effective public policies, it is necessary to profile the vulnerability of this population group, and not only the prevalence or incidence of the pathogens involved in the STIs that affect them.
This study will enable the collection of valuable data for the implementation of public policies aimed at improving the quality of life of police officers, as it addresses the vulnerability issues of this population, encompassing analytical dimensions such as individual/behavioral, social, and programmatic. The focus will be on what should be considered, for example, as motivation in the individual/behavioral dimension, gender relations in the social dimension, access to services in the programmatic dimension, among others.
Objectives
This systematic review and meta-analysis is aimed to describe the vulnerability of police officers to STIs, as well as the associated factors.
METHODS AND ANALYSIS
Study registration
This systematic review protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO) CRD42023422458; https://www.crd.york.ac.uk/PROSPERO/).
Study design
This systematic review and meta-analysis protocol follows the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines.16
Criteria for considering studies for the review and meta-analysis
Inclusion criteria
Original studies addressing police officers and STIs, published in Portuguese, English, or Spanish from January 1, 2000 until August 30, 2023, available free of charge (open access) in full and in original format, regardless of their nature (field research, documentary with secondary data, among others), encompassing in the title or abstract the descriptors defined for the search.
Exclusion criteria
Non-human studies, reviews, theses, editorials, letters, manuals, conference abstracts (grey literature), articles without methods section such as narrative reviews and documental analysis, and studies in duplicate.
Question (PECO)
The definition of the research problem was based on the acronym PECO (P – population; E – Exposure; C – Comparator; O – Outcomes.17 Thus, this protocol has the following guiding question: Are police officers more vulnerable to being infected by STIs and are there factors associated with such vulnerability?
Type of population
Police officers, without restrictions on gender, age, or race.
Type of exposure
Being a police officer.
Type of comparator
General population.
Types of outcome measures
Primary outcomes
Identify the vulnerability of police officers to STIs based on the prevalence and incidence of these diseases compared to the general population.
Secondary outcomes
Identify and/or enumerate significant variables associated with STIs in police officers.
Search methods for identification of studies
Electronic searches
Electronic searches will be conducted in the following six databases: Banco de Dados em Enfermagem (BDENF), Latin American and Caribbean Literature in Health Sciences (LILACS), National Library of Medicine – National Institutes of Health (NIH), Scientific Electronic Library Online (SciELO), SciVerse Scopus, and Web of Science, via Coordination for the Improvement of Higher Education Personnel (CAPES) by accessing the Federated Academic Community (CAFe).
The search strategies will be carried out using Health Sciences Descriptors (DeCS) and Medical Subject Headings (MeSH), with macro-descriptors and synonyms for better coverage of results, in Portuguese, English, and Spanish. The Boolean operators “AND” and “OR” will be used to combine the elements defined by the PECO acronym. The search strategy will be adapted according to each database, maintaining approximation between the controlled and uncontrolled descriptors. The following controlled descriptors will be used: Polícia [Title/Abstract] OR Police [Title/Abstract] OR Policia [Title/Abstract] AND Infecções Sexualmente Transmissíveis [Title/Abstract] OR [Title/Abstract] OR Sexually Transmitted Diseases [Title/Abstract] OR Infecciones de Transmisión Sexual [Title/Abstract] AND Vulnerabilidade em Saúde [Title/Abstract] OR Health Vulnerability [Title/Abstract] OR Vulnerabilidad en Salud [Title/Abstract] AND Comportamento Sexual [Title/Abstract] OR Sexual Behavior [Title/Abstract] OR Comportamiento Sexual [Title/Abstract]. The following uncontrolled descriptors (synonyms) will be employed: Agentes para Cumprimento das Leis [Title/Abstract] OR Law Enforcement Agents [Title/Abstract] OR Agentes Encargados de Hacer Cumplir la Ley [Title/Abstract] AND Doenças Sexualmente Transmissíveis [Title/Abstract] OR Sexually Transmitted Diseases [Title/Abstract] OR Enfermedades de Transmisión Sexual [Title/Abstract] OR Doenças Venéreas [Title/Abstract] OR Venereal Diseases [Title/Abstract] OR Enfermedades venéreas [Title/Abstract] AND Vulnerabilidade e Saúde [Title/Abstract] OR Vulnerability and Health [Title/Abstract] OR Vulnerabilidad y Salud [Title/Abstract] AND Atividade Sexual [Title/Abstract] OR Sexual Activity [Title/Abstract] OR Actividad sexual [Title/Abstract] AND Atração Sexual [Title/Abstract] OR Sexual Attraction [Title/Abstract] OR Atracción Sexual [Title/Abstract] AND Orientação Sexual [Title/Abstract] OR Sexual Orientation [Title/Abstract] OR Orientación Sexual [Title/Abstract] AND Preferência Sexual [Title/Abstract] OR Sexual Preference [Title/Abstract] OR Preferencia Sexual [Title/Abstract].
Selection of studies
In July and August 2023, two independent researchers will blindly assess the titles and abstracts of the studies selected according to the eligibility criteria and, subsequently, will use the Rayyan software18 to identify and remove duplicates. In cases of doubt about the inclusion/exclusion criteria, the text will be read in full. Any disagreement will be discussed by consensus with a third reviewer. The PRISMA statement guidelines16 will be used to outline the phases of electronic searches (Figure 1, Table 1).
Data extraction
The data extraction procedure will follow the recommendations provided in Chapter 7 of the JBI Manual for Evidence Synthesis,19 and the data will be fed into a spreadsheet prepared by the authors using Microsoft Excel Spreadsheet Software 365®. The following information will be collected from each study: authors, year, journal, details of methods (study design, participants, sample, data collection procedure, among others), dependent variables studied (findings and measures used to obtain results), and methods applied for data analysis.
Quality assessment and risk of bias
For the selection of abstracts, the level of scientific evidence of the studies will be independently analyzed by two reviewers according to the classification system of the Agency for Healthcare Research and Quality,20 as recommended by the Cochrane criteria,21 22 and also based on the completeness of the PRISMA checklist16.
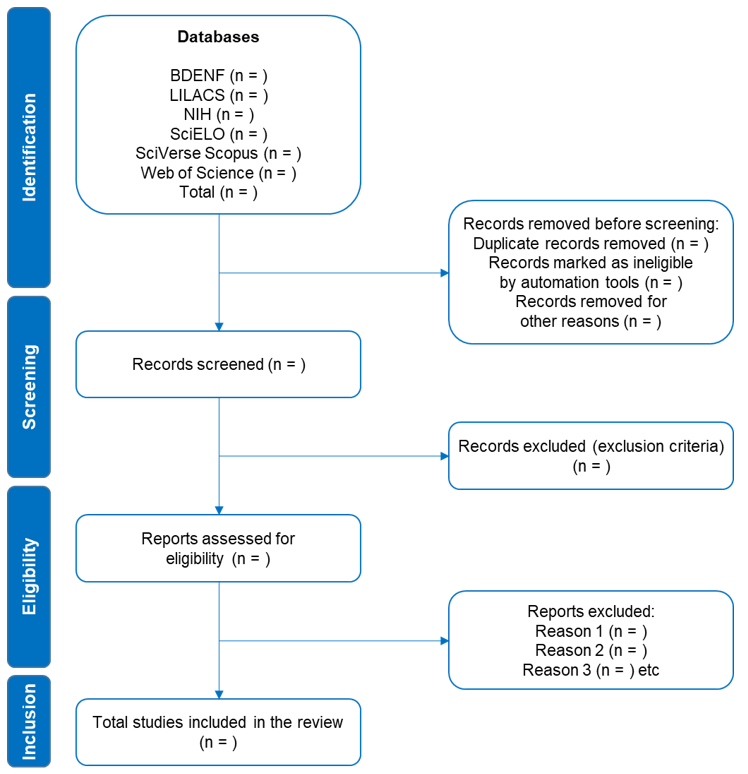
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.16
BDENF, Banco de Dados em Enfermagem; LILACS, Latin American and Caribbean Literature in Health Sciences; NIH, National Library of Medicine – National Institutes of Health; SciELO, Scientific Electronic Library Online.
Table 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) item checklist.16
Section and topic Item # Checklist item Location where item is reported Title Title 1 Identify the report as a systematic review. 1 Abstract Abstract 2 See the PRISMA 2020 for Abstracts checklist (table 2). 1 Introduction Rationale 3 Describe the rationale for the review in the context of existing knowledge. 3 Objectives 4 Provide an explicit statement of the objective(s) or question(s) the review addresses. 4 Methods Eligibility criteria 5 Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. 4 Information sources 6 Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. 6 Search strategy 7 Present the full search strategies for all databases, registers and websites, including any filters and limits used. 6 Selection process 8 Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. 6 Data collection process 9 Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. 7 Data items 10a List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. 5, 6 10b List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. NA Study risk of bias assessment 11 Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. 7, 8 Effect measures 12 Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. NA Synthesis methods 13a Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). 12 13b Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. 12 13c Describe any methods used to tabulate or visually display results of individual studies and syntheses. 12 13d Describe any methods used to synthesise results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. 12 13e Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). 12 13f Describe any sensitivity analyses conducted to assess robustness of the synthesised results. NA Reporting bias assessment 14 Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). 7 Certainty assessment 15 Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. 12 Results Study selection 16ª Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram (see fig 1). NA 16b Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. NA Study characteristics 17 Cite each included study and present its characteristics. NA Risk of bias in studies 18 Present assessments of risk of bias for each included study. NA Results of individual studies 19 For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. NA Results of syntheses 20a For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. NA 20b Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. NA 20c Present results of all investigations of possible causes of heterogeneity among study results. NA 20d Present results of all sensitivity analyses conducted to assess the robustness of the synthesised results. NA Reporting biases 21 Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. NA Certainty of evidence 22 Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. NA Discussion NA Discussion 23a Provide a general interpretation of the results in the context of other evidence. NA 23b Discuss any limitations of the evidence included in the review. NA 23c Discuss any limitations of the review processes used. NA 23d Discuss implications of the results for practice, policy, and future research. NA Other information Registration and protocol 24a Provide registration information for the review, including register name and registration number, or state that the review was not registered. 4 24b Indicate where the review protocol can be accessed, or state that a protocol was not prepared. NA 24c Describe and explain any amendments to information provided at registration or in the protocol. NA Support 25 Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. 13 Competing interests 26 Declare any competing interests of review authors. 13 Availability of data, code, and other materials 27 Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. NA
Three different tools will be used to assess meta-bias such as publication bias and result reporting bias. If 10 or more studies are available, the potential for publication bias will be explored by constructing funnel plots. The Begg and Mazumdar test23 as well as the Egger test24 25 will be used to assess small study effects. Finally, if p ≥ 0.05, we will consider that the study is free from publication bias.
Data analysis
In cases the Review Manager statistical software is inadequate for the analyses, Stata 14 software will be used. To assess heterogeneity between studies, I² statistics will be evaluated, applying random statistics or random proportions (univariate), depending on heterogeneity, with a 95% confidence interval (95%CI) for pooled estimates of the three results of this systematic review. Furthermore, the results will be analyzed using crude mean differences.
The results will be displayed in a forest plot. Meta-analyses will be conducted using the random effects method, because the confidence intervals for the mean effect of the intervention will be wider and the corresponding claims of statistical significance will be more conservative.26 27
ETHICS AND DISSEMINATION
Since this protocol is based on published data, the information used is anonymized, and individuals’ rights are not infringed, it does not require ethics approval. The findings dissemination will follow the PRISMA statement guidelines.16 The results will be published in peer-reviewed journals and presented at scientific conferences and also to workers’ health management systems registered in the public security system in Brazil. In case of any changes to this protocol, they will be registered in PROSPERO as they occur and properly documented in the final publication.
Potential limitations
This study did not receive any financial support and therefore it is limited to open access articles. Furthermore, an important limitation is the scarcity of publications dedicated to the theme addressed, demanding new studies and innovative health care proposals in the context of public safety.
DISCUSSION
To the best of our knowledge, this study is the first systematic review and meta-analysis aiming to analyze the vulnerability of police officers to acquiring STIs. It is believed that the findings will contribute to proposals for preventive, multidisciplinary, and intersectional interventions for future mapping, specialized services, and monitoring of these workers who are extremely vulnerable to STIs.
We hope that this review will be useful to awaken the critical eye of health managers and professionals, considering the institutions’ social commitment, and glimpsing the possibility of developing itinerant health policies for this group that still lacks care.
Contributors MAR, LBF, AFCLB, ACSP and MAM conceived the study, designed the study protocol, and drafted the manuscript. MAR and MAM will perform the searches, data extraction, and analysis for the systematic review and meta-analysis, and any disagreement will be discussed with AFCLB and ACSP. All the authors will provide oversight of the searches, data extraction, and analysis. MAM will provide statistical input for data analysis. All authors contributed to the writing of this manuscript as well as read and approved its final version.
Funding This work received no specific funding.
Competing interest statement The authors declare no conflicts of interest.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- World Health Organization. Sexually transmitted infections (STIs) (updated August 2022). Geneva, Swizerland: World Health Organization, 2022. https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(STIs) (accessed 25 May 2023).
- Wihlfahrt K, Günther V, Mendling W, et al. Sexually transmitted diseases-an update and overview of current research. Diagnostics 2023;13:1656.
- United Nations. Transforming our world: the 2030 Agenda for Sustainable Development. New York, NY, United States: United Nations, 2015. https://documents-dds-ny.un.org/doc/UNDOC/GEN/N15/291/89/PDF/N1529189.pdf?OpenElement (accessed 2 June 2023).
- Wu X, Zhou X, Chen Y, et al. The impact of COVID-19 lockdown on cases of and deaths from AIDS, gonorrhea, syphilis, hepatitis B, and hepatitis C: interrupted time series analysis. JMIR Public Health Surveill 2023;9:e40591.
- Hojilla JC, Sarovar V, Lam JO, et al. Sexually transmitted infection screening in key populations of persons living with HIV. AIDS Behav 2023;27:96–105.
- Ravalihasy A, Ante-Testard PA, Kardas-Sloma L, et al. Quantitative methods used to evaluate impact of combination HIV prevention intervention: a methodological systematic review. AIDS Behav 2023;1–11.
- Hopkins D, Wilson C, Allard R. Sexually transmitted infections in US military women: A scoping review 2000–2018. Womens Health Issues 2021;31 Suppl 1:S43–52.
- Somsri M, Oransathid W, Vesely B, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae in adult patients seeking care at military hospitals in Thailand from 2014 to 2020. Mil Med 2022;usab549.
- Futino RS, Delduque, MC. Saúde mental no trabalho de segurança pública: estudos, abordagens e tendências da produção de conhecimento sobre o tema. Cad Ibero-Am Dir Sanit 2020;9:116–34. (In Portuguese).
- Zemke JN, Sanchez JL, Pang J, et al. The double-edged sword of military response to societal disruptions: A systematic review of the evidence for military personnel as pathogen transmitters. J Infect Dis 2019;220:1873–84.
- Campbell WR, Jahan M, Bavaro MF, et al. Primary care of men who have sex with men in the US Military in the post-Don’t Ask, Don’t Tell era: A review of recent progress, health needs, and challenges. Mil Med 2017;182:e1603–11.
- Wong ML, Lubek I, Dy BC, et al. Social and behavioural factors associated with condom use among direct sex workers in Siem Reap, Cambodia. Sex Transm Infect 2003;79:163–5.
- Clark MM, Warren BA, Hagen PT, et al. Stress level, health behaviors, and quality of life in employees joining a wellness center. Am J Health Promot 2011;26:21–5.
- Korzeniewski K, Juszczak D, Paul P. Sexually transmitted infections in the military environment. Int Marit Health 2020;71:207–12.
- Singkun A, Kallawicha K, Yamarat K. Sexual knowledge based on Islamic values and sexual risk behaviors of HIV/STIs among Thai Muslim army conscripts: A cross-sectional study. Belitung Nurs J 2022;8:431–7.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021;372:n71.
- Morgan RL, Whaley P, Thayer KA, et al. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018;121:1027–31.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan — a web and mobile app for systematic reviews. Syst Rev 2016;5:210.
- Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis (updated 8 June 2022). Adelaide, Australia: Joanna Briggs Institute, 2020. https://jbi-global-wiki.refined.site/space/MANUAL/4687372/Chapter+7%3A+Systematic+reviews+of+etiology+and+risk (accessed 6 June 2023).
- Stetler CB, Morsi D, Rucki S, et al. Utilization-focused integrative reviews in a nursing service. Appl Nurs Res 1998;11:195–206.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
- Leeflang MMG, Deeks JJ, Takwoingi Y, et al. Cochrane diagnostic test accuracy reviews. Syst Rev 2013;2:82.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101.
- Stuck AE, Rubenstein LZ, Wieland D. Bias in metaanalysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998;316:469.
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.
1Nursing School, Universidade Estadual de Goiás, Ceres, GO, Brazil, e-mail: meillyne.reis@ueg.br, Orcid: https://orcid.org/0000-0001-5953-4398.
2Municipal Secretary of Health, Goiânia, GO, Brazil, e-mail: laisbarbaraferreira@gmail.com, Orcid: https://orcid.org/0000-0002-8804-1960.
3Nursing School, Universidade Federal de Jataí, Jataí, GO, Brazil, e-mail: aninhacarvalholima@gmail.com, Orcid: https://orcid.org/0000-0002-3922-2837.
4Nursing School, Universidade Federal de Jataí, Jataí, GO, Brazil, e-mail: ana_claudia_souza@ufj.edu.br, Orcid: https://orcid.org/0000-0003-2099-2257.
5Graduate Program in Nursing, Nursing School, Universidade Federal de Goiás, Goiânia, GO, Brazil, e-mail: marcosmatos@ufg.br, Orcid: https://orcid.org/0000-0001-8643-7032.
Correspondence to Meillyne Alves dos Reis; meillyne.reis@ueg.br
