REGISTRO DOI: 10.5281/zenodo.12699841
Luciana de Macedo Mello1
Scarlath Ohana Penna dos Santos2
Rodiney Pinheiro Denevitz3
Isabella Cristina Morales4
Márcia Faes5
Priscila Del Aguila da Silva6
Marcus Antonio Rossi Feliciano7
Guilherme de Souza Vieira8
Milene Botelho Bartholazzi9
Julia Menezes Machado10
Stefany Martins de Almeida11
Hassan Jerdy Leandro12
Fernanda Antunes13
André Lacerda de Abreu Oliveira14*
Abstract
Inflow occlusion consists of promoting circulatory arrest capable of allowing an intracardiac approach for a brief period. The technique’s tolerance in cardiac patients is very short, with recommendation of two minutes at most. Cardiac and neurological complications can occur due to poor systemic and cerebral perfusion. However, there are still no data in the literature on the damage caused to the kidneys by the occlusion technique. The purpose of the present study was to evaluate, through contrast ultrasonography with microbubbles, the hemodynamic and structural repercussions on the renal system of pigs that underwent induced ischemia and reperfusion by the inflow occlusion technique for two minutes. Based on the results, the contrast-enhanced ultrasound was efficient in identifying and evaluating renal perfusion, suggesting that the increase in maximum numeric pixel value (NPV) during cortical wash-in is related to the reperfusion process. The short time of ischemia was not enough to promote injury or significant changes, to the point of altering microperfusion and promoting cell necrosis, but it was related to hydropic degeneration found in renal tissues, a histopathological finding that precedes necrosis, suggesting that occlusions longer than two minutes may be related to significant renal injury.
Introduction
Venous inflow occlusion is a technique for open heart surgery where the venous flow be stopped during the operation. Since it results in complete circulatory arrest, it allows a limited time to perform cardiac procedures. (Griffiths 2010). Cardiac and neurological complications can occur due to poor systemic and cerebral perfusion, particularly in occlusions lasting longer than three minutes (Gokalp et al. 2011). However, there are still no data in the literature on the damage caused to the kidneys by the inflow occlusion technique.
Venous inflow occlusion in the heart was the most commonly used technique in cardiac surgery before the cardiopulmonary bypass (CPB) era, being routinely indicated for correction of cardiac defects such as pulmonary or aortic valve stenosis, correction of interatrial septal communication, and removal of thrombi and intracardiac foreign bodies. Although CPB appears to be superior, its approaches are subject to technical complications inherent to tissue damage and embolic events during cannulation, in addition to perioperative complications secondary to the inflammatory process triggered by cardiopulmonary bypass, being contraindicated for some critical patients, making inflow occlusion an alternative technique still indicated today (Bolbadia 2006; Gokalp et al. 2011).
The duration of occlusion tolerated by patients with pre-existing cardiovascular disease is likely to be significantly shorter, so a safety margin should be incorporated in the plan to allow for approaches in case of complications. Consequently, for occlusion of the flow of normothermic heart disease patients, the ideal period is two minutes or less (Griffiths 2010). Thus, the inflow occlusion technique is safe for short periods in selected patients operated by experienced teams.
The kidneys play a central role in regulating blood pressure, so reduced cardiac output or hypotension causes decreased renal perfusion. Ischemia can occur when there is restriction of blood supply to a certain tissue, while reperfusion occurs when there is restoration of blood flow, with resumed oxygenation (Lamby et al. 2017). Renal ischemia can occur in the anesthetic surgical routine in different situations, such as cardiac arrest with resuscitation, renal artery revascularization, renal transplantation, unilateral nephrectomy, and in common postoperative complications of cardiac surgery (Kramer et al. 2015). Kidney damage is defined as a progressive decrease in the glomerular filtration rate, accumulation of nitrogenous residues such as urea and creatinine, and inability to regulate homeostasis (Lynch et al. 2008), all of which can occur in these procedures. However, measurement of serum creatinine levels lacks the necessary sensitivity and specificity for early detection (Obermu et al. 2014). The increase in creatinine concentration can occur over 48 hours (Mahoney et al. 2014).
Contrast-enhanced ultrasound (CEUS) uses the technology of intravenous injection of gas microbubbles. The microbubble contrast agent, sulfur hexafluoride, can be detected in the circulation for several minutes (Bouakaz & Jong 2007). Microbubbles are formed by a complex gas with low solubility, and are pure intravascular agents (Harvey 2015), with properties similar to those of red blood cells. Their presence can be used to quantify the renal arterial blood flow in real time (Granata et al. 2009), and does not present nephrotoxicity, allowing the dynamic detection of blood flow both in the renal macro and microvasculature, making them an excellent ally in early dynamic evaluations (Yue et al. 2016).
The present study aims to evaluate the hemodynamic and structural repercussions on the renal system of individuals undergoing temporary circulatory arrest (inflow occlusion) through contrasted ultrasonography with microbubbles in comparison with histopathological findings.
Materials and Methods
The present study was conducted in full compliance with all applicable research ethics and animal welfare regulations, under a general standing authorization by the Animal Use Ethics Committee (CEUA) of the Darcy Ribeiro Norte Fluminense State University, under the protocol number: 424451, according to Brazilian Law 11,794/08. The study design was experimental using a young swine model (14 – 20 kg). The animals were kept throughout the preoperative period with adequate handling conditions, in accordance with animal welfare standards. They were kept at the experimental unit in group stall with no more than two pigs in each space, with feed and water available ad libitum.
For surgery, the animals were contained manually and received a pre-anesthetic regimen consisting of acepromazine at a dose of 0.05 mg/kg, associated with ketamine hydrochloride at 10 mg/kg and midazolam at 0.3 mg/kg, administered in the semimembranosus muscle. After obtaining the desired anesthetic effect, the right cephalic vein was accessed with a silicone intravenous catheter. Immediately after access correction, blood samples were collected for cell counts.
In the blood work, the erythrogram, white blood cell count and platelet count were analyzed. Serum biochemistry evaluations were conducted of urea, creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total protein, albumin, globulin and phosphorus.
The sedated animals were manually restrained during the imaging exam, without the need for replication of the pre-anesthetic medication. For contrast enhanced harmonic imaging, a 5-12 MHz frequency linear transducer and a harmonic imaging system were used (Logiq V2, GE Medical Systems, China).
The kidneys were also evaluated by conventional B-mode ultrasonography to rule out possible focal or diffuse abnormalities, in addition to the evaluation by vascular Doppler imaging in order to verify the integrity of the renal, interlobar, arcuate, and interlobular arteries and to ascertain the presence or absence of areas of vascularization. Vascularization-specific Doppler analysis was performed after localization of the renal artery (Yi et al. 2012). A minimum of three subsequent waves were obtained to conclude the assessment. The parameters studied were: systolic velocity (SV, cm / s); diastolic velocity (DV, cm/s); resistivity index (RI = (maximum velocity – minimum velocity) /maximum velocity), and blood flow pattern (high, intermediate or low resistivity) (Le Dorze et al. 2012).
For contrast-enhanced ultrasound, a sulfur hexafluoride contrast agent (SonoVue; Bracco SpA, Milan, Italy) was administered through the cephalic vein at a dose of 0.06ml/kg after constant agitation. A bolus of 5 mL of saline was performed to allow optimization of image settings and verification of uniform perfusion of the kidney. The images were evaluated in relation to the presence or absence of filling contrast, filling homogeneity (homogeneous or heterogeneous). In addition, the renal vascular filling times from contrast injection to the beginning of organ perfusion were determined: wash-in (entry time) (Figure 1), contrast peak (enhancement) (Figure 2) and wash-out (exit time) (Figure 3), in the different renal phases (vascular, cortical and medullary).
The parameters of the ultrasound equipment were constant for all exams. The microbubble perfusion process and dynamic enhancement of each lesion were observed in real time and continuously recorded by the equipment during the period when the right kidney no longer presented contrast (Yi et al., 2012).
For quantitative evaluation of the echotextural characteristics of the tissues of interest, computerized image analysis was performed using commercial image analysis software (Image ProPlus®, Media Cybernetics Inc., San Diego, California, USA). This software assigns values from 0 (black color) to 255 (white color) and provides an indicator of tissue echogenicity. An area of primary interest was defined covering the regions of the renal cortex and medulla, separately, and six points of regions of interest (ROI) were designed, each with diameter of 20 mm, to calculate mean, maximum and minimum numeric pixel values (NPV). For the selection of ROI, special care was taken to exclude areas with artifacts and non-parenchymal tissue.
The surgical procedure was performed in the High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit of Darci Ribeiro Norte Fluminense State University (UEA/UENF). The venous access enabled ringer-lactate infusion through a controlled dose of 10 ml/kg/hr. Anesthetic induction was performed with the association of propofol at a dose of 5 mg/kg, associated with thiopental at a dose of 12,5 mg/kg. Then intubation was performed with a tracheal tube (number 5.5) connected to an inhalation anesthesia machine through the Bain circuit. The animals were maintained under anesthesia with isoflurane and 100% oxygen inhalation. For analgesic control, continuous intravenous infusion was performed of ketamine hydrochloride at a dose of 0.6 mg/kg/h and intercostal blockade with lidocaine hydrochloride without vasoconstrictor at a dose of 5 mg/kg.
During the trans-operative period, monitoring was performed using a multiparametric monitor (Digicare LifeWindow LW9x Vet®, United States), the heart rate (HR) and oxyhemoglobin saturation (SPO2) were evaluated by attaching the sensor to the lower lip of the animals, and blood pressure was measured using a non-invasive method.
The animals underwent left lateral thoracotomy in the fifth intercostal space, followed by dissection of anatomical planes to access the chest cavity, and the lung lobes were dorsally retracted to open the caudal mediastinum. After the incision in the caudal mediastinum and careful dissection of the structures, the caudal vena cava, cranial vena cava and azygos vein were identified and individualized. The pericardium was incised along the cardiac axis. In sequence, Satinsky clamps were positioned to respectively occlude the azygos vein, caudal vena cava and cranial vena cava. The clamping of vessels was maintained for two minutes, and after this time of circulatory arrest, the Satinsky clamps were released, enabling cardiac and systemic reperfusion.
A chest tube was properly positioned between the 7th and 8th intercostal space. Thoracoraphy was performed using number 5 polyester thread for approximation of the ribs, by three stitches in a sultan pattern. Subsequently, a simple continuous suture was performed to approximate the incised thoracic musculature, as well as the skin tissue. At the end of the procedure, negative pressure was established after removal of residual air through the chest tube.
Respecting the ethical principles of animal experimentation, immediately after the experiment, the animals were euthanized. For this purpose, they were subjected to deep general anesthesia with propofol followed by intravenous administration of 19.1% potassium chloride at dose of 100 mg/kg.
At the end of the trial, the kidneys were collected for histopathological analyses to evaluate cell lesions, such as edema, hydropic degeneration, congestion, infarction and hemorrhage. The histopathological analyses were carried out at the university’s Veterinary Anatomopathological Diagnostic Laboratory of the Animal Pathology Sector.
In the statistical study, parametric analyses were performed between pre-surgical and post-surgical times. Data were tabulated and prevented using GraphPad Prism version 6.0 The Mann-Whitney and Kolmogorov-Smirnov tests were performed. The groups were compared by the Student t-test. The significance level of all tests was p < 0.05.
Results
In the analysis of the erythrogram before and after the operation, no statistical differences were found (p>0.05) for the number of red blood cells, mean corpuscular hemoglobin concentration (MCHC) and fibrinogen. For the other variables evaluated in the blood count, statistical differences were found. The globular volume (VG) was higher (p<0.002) in the preoperative period (30.0 ± 0.7%) compared to the postoperative period (25.3 ± 0.5%). Hemoglobin concentration was also higher (p<0.001) in the preoperative period (10.0 ± 0.2 g/dl) in comparison with the postoperative period (8.3 ± 0.1 g/dl). For MCHC, a higher value was found in the postoperative period than the preoperative period (50.4 ± 3.6 fI and 49 ± 3.6 fI, respectively). Higher plasma protein concentrations were found in the preoperative period (6.5 ± 0.1 g/dl) than in the postoperative period (5.9 ± 0.05 g/dl).
In the biochemical analysis, no statistical differences were found (p>0.05) for the variables: urea, ALT, FA, GGT, PTN, albumin and globulin. Creatinine was higher (p<0.009) during the preoperative period (0.95 ± 0.01 mg/dl) than postoperative period (0.79 ± 0.03 mg/dl). For the variable AST, the postoperative values were higher (p<0.03) than the preoperative values (50 ± 19.1 UI/L and 23 ± 2.5 UI/L, respectively). Higher means (p<0.002) were found for phosphorus in the postoperative period (8.48 ± 0.28 mg/dl) compared to the preoperative period (6.35 ± 0.31 mg/dl).
In the B-mode ultrasound evaluation, no structural changes were found in any animal: the kidneys’ shape and dimensions were preserved, along with regular contours and cortical-medullary differentiation.
In the evaluation in Doppler mode, all parameters remained uniform, and the RI remained within the standards of the species. In the evaluation performed using the microbubble technique, the only parameter that showed statistical difference (p<0.02) was the wash-in in the cortical bone, which had the highest postoperative mean (136.6 ± 2.3 pixels) in relation to the preoperative period (12.30 ± 4.1 pixels) (Figure 4).
Kidney histopathology consisted of a diffuse severe degenerative process. The epithelial cells of the cortical and medullary renal tubules were enlarged. The cytoplasm was eosinophilic, rarefied and finely to moderately vacuolated. The nucleus was normal, central to paracentral, basophilic and vesicular, predominantly with euchromatin and small round nucleoli. These findings are compatible with moderate to severe hydropic degeneration.
Discussion
Changes in physiological variables at the end of the experiment were not clinically impactful, despite showing an increase in hematocrit and hemoglobin. Medications commonly used for anesthesia can significantly alter the oxidative state of blood cells. This mechanism can contribute to the immunological suppression that occurs transiently in the initial postoperative period (Delogu et al. 2004). Changes in erythrogram values can result from several mechanisms, including stress, cell marginalization and sequestration by some organs (Wilson et al. 2004). The results were also similar to those obtained by Kiliç (2008), who used the association of ketamine with detomidine and midazolam administered intravenously in cattle and found a decrease in hematocrit and hemoglobin over the period of anesthetic action. Similarly, Tiburcio et al. (2014) also found a reduction in hemoglobin values after intravenous injection of acepromazine, detomidine and xylazine in cattle.
In our study, there was an increase in phosphorus values between the the pre- and postoperative period, but they remained within the reference values (5.3 – 9.6 mg/dl) (González & Silva 2008), and could not be considered a biomarker for acute kidney injury in this work.
Acute kidney injury is a common complication after cardiac surgery. Pathophysiology includes decreased renal perfusion, oxidative stress, hypothermia and inflammatory response (Zappitelli et al. 2015).
According to B-mode ultrasonography, all animals had homogeneous echotexture and preserved echogenicity in both kidneys. The corticomedullary relationship was well defined, with no evidence of obstructive processes, dilatation or nephrolithiasis. This assessment was important to exclude animals with possible nephropathies.
In the Doppler renal vascular evaluation, there were no alterations in the parameters of the renal artery blood flow in the preoperative period. Some methods to assess renal function are effective, but complex and invasive, which restricts their use in clinical settings (Melo et al. 2006). The use of Doppler blood flow examination in organs such as the kidneys allows the detection of blood flow abnormalities, contributing to a more accurate diagnosis (Szatmári et al. 2002; Melo et al. 2006).
Bragato et al. (2017) evaluated the B-mode and Doppler ultrasound to investigate chronic kidney disease, and observed in the B-mode ultrasound images an increase in cortical echogenicity, loss of corticomedullary differentiation, reduction in renal volume and irregular renal contour. Through the Doppler mode, the authors evaluated the perfusion, which was greater in chronic kidney injuries. The authors concluded that these tests are important for renal assessment, both in diagnosis and in prognosis.
Doppler ultrasound is important to assess conditions that alter the perfusion of the renal parenchyma (Baltazar et al. 2016), with the advantage that renal alterations can be quantified by calculating the RI and pulsatility index (PI) (Carvalho et al. 2009). The measurement of systolic and diastolic flow velocities of the renal arteries allows the calculation of the RI, enabling the detection of renal pathologies associated with changes in renal vascular resistance (Rivers et al 1997). Meola et al. 2016 suggested that RI and Doppler-based renal PI are indicators in monitoring renal function
In our study, the mean values of RI (0.72) and IP (1.4) were acceptable for the species, according to Rawashdeh et al. (2000), who evaluated the resistance index in an experimental study of the normal range in pigs where RI values ranged from 0.48–0.85, with mean value of 0.70. Mei et al. (2015) observed that the RI increased and the PI decreased, after cardiac arrest with ventricular fibrillation in pigs that had acute kidney injury. However, Cole et al (2000), studying human patients with partially obstructed kidneys, found a statistical difference, with increased RI five days after surgery.
The introduction of CEUS during the last decade opened the possibility for noninvasive dynamic studies of macrocirculation and microcirculation in various regions of the body, including the kidneys (Jiménez et al. 2008). In our study, we only observed a difference in the NPV of the maximum wash in the cortical bone. Unlike the results reported by Brabrand et al. (2014), who showed through microbubbles a decrease in renal perfusion after a period of hypoxia in piglets, we observed that cortical and medullary flows were affected differently by hypoxia. An increase was found of mean time to peak and mean medullary transit time and a reduction in cortical peak intensity.
In a study by Claudon et al. (1999), contrast ultrasound was used in swine to assess renal flow with perfluorohexane nitrogen contrast and surfactant coating in acute urinary obstruction. The authors reported a change in renal flow, with a significant decrease in the peak enhancement in the medullary in relation to the peak enhancement in the cortical bone. In our study, the wash-in (mean and minimum NPV), enhancement peak and wash-out (maximum, mean and minimum NPV) did not differ, showing satisfactory perfusion. During the perioperative period, the animals maintained HR (95 bpm) and medium blood pressure (74 mmHg) within normal values for the species (Thrall et al. 2007), which probably contributed to hemodynamic stability without considerable change in renal vascular resistance and change in renal blood flow.
Mahoney et al. (2014), in a study with microbubbles, evaluated the renal perfusion of healthy rats and animals that had ischemia-reperfusion injury. The authors observed a reduction in wash-in and peak enhancement, compared to control animals, followed by progressive recovery of vascular function within 48 hours. Additionally, Quaia et al. (2006) detected focal renal perfusion lesions with microbubbles in rabbits after injecting microbubble contrast into the abdominal aorta, indicating the safety and accuracy of the examination. In our work, the NPV suffered little variation, indicating that the evaluated renal structure remained uniform. The maximum intensity of the enhancement of the cortical wash-in showed areas that were more contrasted in the postoperative period. However, this does not mean there was impairment of the general perfusion of the renal parenchyma.
The significant presence of hydropic degeneration observed in the samples was common in all treated animals. Degeneration occurred in both kidneys. Silva et al. (2020) carried out a study for cardiac evaluation by videothermometry in animals undergoing inflow occlusion. Their main histopathological findings in the analyzed cardiac samples were the presence of hydropic degeneration in all cardiac chambers. They emphasized that the cellular degenerative process is linked to ischemic and reperfusion processes, leading to the accumulation of intracellular fluid, so that hypoxia is the most important cause of hydropic cell degeneration (Zachary 2018; Jerdy et al. 2020).
Jennings and Steenbergen (1998), in a study of ischemia and reperfusion, reported a 21% increase in total tissue water after two minutes of reperfusion and 43% after 20 minutes. Jerdy (2020) conducted a study with sea turtles with a history of drowning, and observed that even after being rescued and apparently stabilized, they died, with hydropic degeneration of hepatocytes, renal tubular cells and cardiac myocytes being observed, in addition to congestion and necrosis related to prolonged ischemia. In our study, the presence of hydropic degeneration was observed in all animals undergoing a two-minute period of circulatory arrest by the inflow occlusion technique, but no signs of cell necrosis were observed, which demonstrates good prognosis for patients undergoing hypoxia for a brief period. The prognosis of hypoxic patients depends on the number of cells affected and the immediate importance of the loss of cell function (Zachary, 2018), so the longer the time of ischemia and cell stress, the greater the injury and tissue impairment.
Acknowledgments
This work was financially supported through grants from the CAPES (Office to Coordinate Improvement of Higher Education Personnel) and Animal Science Graduate Program of Darcy Ribeiro Norte Fluminense State University, where this study was part of the first author’s doctoral thesis research.
The authors would like to thank the staff and volunteers at Darci Ribeiro Norte Fluminense State University and collaborators from Federal University of Santa Maria and Julio Mesquita Filho São Paulo State University.
Abbreviations
NPV – Numeric pixel value
CEUS – Contrast-enhanced ultrasound
CEUA – Animal Use Ethics Committee
CPB – Cardiopulmonary bypass
DV – Diastolic velocity
HR – Heart rate
IP – Pulsatility index
MCHC – Mean corpuscular hemoglobin concentration
RI – Resistivity index
ROI – Regions of interest
SPO2 – Oxyhemoglobin saturation
SV – Systolic velocity
UEA – Animal Experimentation Unit
UENF – Darci Ribeiro Norte Fluminense State University
8. Author contributions
LMM assisted with the study design, established the anesthetic protocol, led the anesthetic procedure of the animals and assisted in manuscript preparation; SOPS assisted with the study design, led surgeries and assisted in manuscript preparation; RPD and ICM assisted with the surgical procedures and the manuscript preparation; MF and MBB assisted with the imaging studies and the manuscript preparation; PDAS and MARF assisted with computerized analysis of contrasted images and the manuscript preparation; GSV and JMM assisted in taking care of the animals prior to the surgical procedures and contributed to the manuscript preparation; SMA assisted with laboratory analysis and the manuscript preparation; HJL assisted with the histopathological analysis and the manuscript preparation; FA performed the statistical analysis and assisted in manuscript preparation; ALAO assisted with the study design and participated in manuscript preparation.
9. References
BALTAZAR IP et al. 2016. Comparative B-mode and Doppler renal ultrasonography with histopathological findings in dogs positive for canine visceral leishmaniasis. Microsc Res Tech 79(7): 637-45.
BOBADILLA JL, WIGFIELD CH & CHOPRA PS. 2006. Inflow occlusion pulmonary embolectomy in the modern era of cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery, 131(2): 2006.
BOUAKAZ A & JONG N. 2007. Safety of Ultrasound Contrast Agents. Ultrasound Med Biol 33(2): 187-196.
BRABAND K, LANGE C, EMBELN KE, REINHOLT FP, SAUGSTAD OD, STOKKE ES & MUNKEBY BH. 2014. Contrast-enhanced ultrasound identifies reduced overall and regional renal perfusion during global hypoxia in piglets. Invest Radiol 49(8):540-6.
BRAGATO N, BORGES NC & FIORAVANTI MCS. 2017. B-mode and Doppler ultrasound of chronic kidney disease in dogs and cats. Vet Res Commun 41(4):307-315.
CARVALHO CS, CERRI GG & CHAMMAS MC. 2009. Doppler velocimetric parameters of aorta and renal arteries of Persian cats. Cienc Rural 39(4):1095-100.
CLAUDON M, BARNEWOLT CE, TAYLOR GA, DUNNING PS, GOBET R & BADAWY AB. 1999. Renal blood flow in pigs: Changes depicted with contrast-enhanced harmonic US imaging during acute urinary obstruction. Radiology 212(3): 725-731.
COLE L, BELLOMO R, SILVESTER W & REEVES JH. 2000. A prospective, multicenter study of the epidemiology, management, and outcome of severe acute renal failure in a “closed” ICU system. Am J Respir Crit Care Med 162(1): 191-6.
DELOGU G, ANTONUCCI A, MORETTI S, MARANDOLA M, TELLAN G, SIGNORE M & FAMULARO G. 2004. Oxidative stress and mitochondrial glutathione in human lymphocytes exposed to clinically relevant anesthetic drug concentrations. J Clin Anesth 16(3): 189-94.
GRIFFITHS LG. 2010. Surgery for Cardiac Disease in Small Animals: Current Techniques. Vet Clin North Am Small Anim Pract 40(4): 605-622.
GOKALP O, YUREKLI I, YILIK L, BAYRAK S, GOKTOGAN T, ARIKAN E, YETKIN U & GURBUZ A. 2011. Comparison of inflow occlusion on the beating heart with cardiopulmonar bypass in the extraction of a mass lesion or a foreign body from the right heart. Eur J Cardiothorac Surg 39: 689-692.
GRANATA A, ANDRULLI S, BIGI MC. 2009. Predictive role of Duplex Doppler ultrasonography in the diagnosis of acute renal obstruction in patients with unilateral renal colic. Clin Nephrol 71: 680-6.
GONZÁLEZ FHD & SILVA SC. 2008. Veterinary Clinical Pathology: Introductory Text. Porto Alegre: Universidade Federal do Rio Grande do Sul, 342p.
HARVEY C. 2015. Ultrasound with microbubbles. Cancer Imaging 15(1):1-2.
JERDY H, MASTRANGELLI A, LACERDA P, BALDASSIN P, SCARELLI AC, WERNECK MR & CARVALHO E. 2020. Anoxia Effects in Asphyxiated Green Sea Turtles (Chelonia mydas) Caught in an Artisanal Fishing Net on the Coast of Brazil. J Comp Path 176:67-70.
JENNINGS RB & STEENBERGEN CJ. 1998. Ca2+ion shifts in vivo in reversible and irreversible ischemic injury. In:The Ischemic Heart. Boston: Kluwer Academic Publishers, p.151–76.
JIMÉNEZ C et al. 2008. In Situ Kidney Insonation With Microbubble Contrast Agents Does Not Cause Renal Tissue Damage in a Porcine Model. J Ultrasound Med 27(11): 1607-15.
KILIÇ N. 2008. Cardiopulmonary, biochemical and haematological changes after detomidine-midazolam-ketamine anaesthesia in calves. Bull Vet Inst Pulawy 52(3): 453-456.
KRAMER RS, HERRON CR, GROOM RC & BROWN JR. 2015. Acute Kidney Injury Subsequent to Cardiac Surgery. J Extra-Corpor Technol 47(1): 16-28.
LAMBY P et al. 2017. Effect of iodinated contrast media on renal perfusion: A randomized comparison study in pigs using quantitative contrast-enhanced ultrasound (CEUS). Sci Rep 7(1): 1-10.
LE DORZE M, BOUGLÉ A, DERUDDRE S & DURANTEAU J. 2012. Renal Doppler ultrasound: a new tool to assess renal perfusion in critical illness. SHOCK 37(4):360-5.
LYNCH BA, GAL P, RANSOM JL, CARLOS RQ, DIMAQUILA MAVT, SMITH MS, WIMMER JR JE & IMM MD. 2008. Low-dose aminophylline for the treatment of neonatal non-oliguric renal failure – case series and review of the literature. Journal Pediatr Pharmacol Ther 13(2): 80-87.
MAHONEY M, SORACE A, WARRAM J, SAMUEL S & HOYT K. 2014. Volumetric Contrast-Enhanced Ultrasound Imaging of Renal Perfusion. J Ultrasound Med 33(8): 1427-1437.
MEI X, HANG CC, WANG S, LI CS & YU ZX. 2015. Renal Doppler and Novel Biomarkers to Assess Acute Kidney Injury in a Swine Model of Ventricular Fibrillation Cardiac Arrest. Chin Med J 128(22): 3069-75.
MELO MB, VEADO JCC, SILVA EF, MOREIRA SM & PASSOS LMF. 2006. Renal arteries Dopplerfluxometry: normal systolic and diastolic flow velocities and resistive index values in the main renal arteries. Arq Bras Med Vet Zootec 58(4): 691-693.
MEOLA M, SAMONI S & PETRUCCI I. 2016. Clinical Scenarios in Chronic Kidney Disease: Vascular Chronic Diseases. Contrib Nephrol 188: 81-88.
OBERMU N, WEIPERT C & URBSCHAT A. 2014. Current developments in early diagnosis of acute kidney injury. Int Urol Nephrol 46(1): 1-7.
QUAIA E, D’ONOFRIO M,PALUMBO A, ROSSI S, BRUNI S & COVA M. 2006. Comparison of contrast-enhanced ultrasonography versus baseline ultrasound and contrast-enhanced computed tomography in metastatic disease of the liver: diagnostic performance and confidense. Eur Radiol 16: 1599-1509.
RAWASHDEH YF, MORTENSEN J, HORLYCK A, OLSEN KO, FISKER RV, SCHROLL L & FROKIAER J. 2000. Resistive index: an experimental study of the normal range in the pig. Scand J Urol Nephrol 34(1):10-4.
RIVERS BJ, WALTER PA, LETOURNEAU JG, FINLAYDE, RITENOUR RE, KING VL, O’BRIEN TD & POLZIN DJ. 1997. Dúplex doppler estimation of resistive index in arcuate arteries of sedated, normal female dogs: Implications for use in diagnosis of renal failure. J Vet Intern Med 33: 69-76.
SILVA TOB, VIDAL LWM, CABRAL PGA, COSTA MRM, CADENA SMR, SANTOS JR MB, ANTUNES F & OLIVEIRA ALA. 2020. Interventionist videothermometry: a new model of cardiac ischemia evaluation. BMC Vet Res 16(142): 1-9.
SZATMÁRI V, SÓTONYI P & VOROS K. 2002. Normal duplex doppler waveforms of major abdominal blood vessels in dogs : a review. Vet Radiol Ultrasound 42(2): 93-107.
THRALL MA, WEISER G, ALISSON RW & CAMPBELL TW. 2014. Hematologia e bioquímica clínica veterinária. 2nd ed., São Paulo: Roca, 688 p.
TIBURCIO M, OLIVEIRA MS, MARTINI MV, DIAS LGGG & MATTOS JR E. 2014. Acepromazina, detomidina ou xilazina na sedação em equinos: efeitos hematológicos e bioquímicos. Rev Acad Ciênc Agrár Ambient 12(1): 35-44.
WILSON DV, EVANS AT, CARPENTER RE & MULLINEAUX DR. 2004. The effect of four anesthetic protocols on splenic size in dogs. Vet Anaesth Analg 31: 102-108.
YI K, JI S, KIM J,YOON J & CHOI M. 2012. Contrast-enhanced ultrasound analysis of renal perfusion in normal micropigs. J Vet Sci 13(3): 311-314.
YUE WW, WANG S, XU HX, SUN LP, GUO AH, BO XW, LI XL, ZHAO CK, WANG D & LIU BJ. 2016. Parametric imaging with contrast-enhanced ultrasound for differentiating hepatocellular carcinoma from metastatic liver câncer. Clin Hemorheol Microcirc 64(2):177-188.
ZACHARY JF. (2018). Pathologic basis of veterinary disease, 6th Ed., St. Louis: Mosby, 1476p.
ZAPPITELLI M et al. 2015. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 169(6):583-91.
Legends de figuras e tabelas
Figure 1- Right kidney at the wash in moment, after microbubble contrast administration.
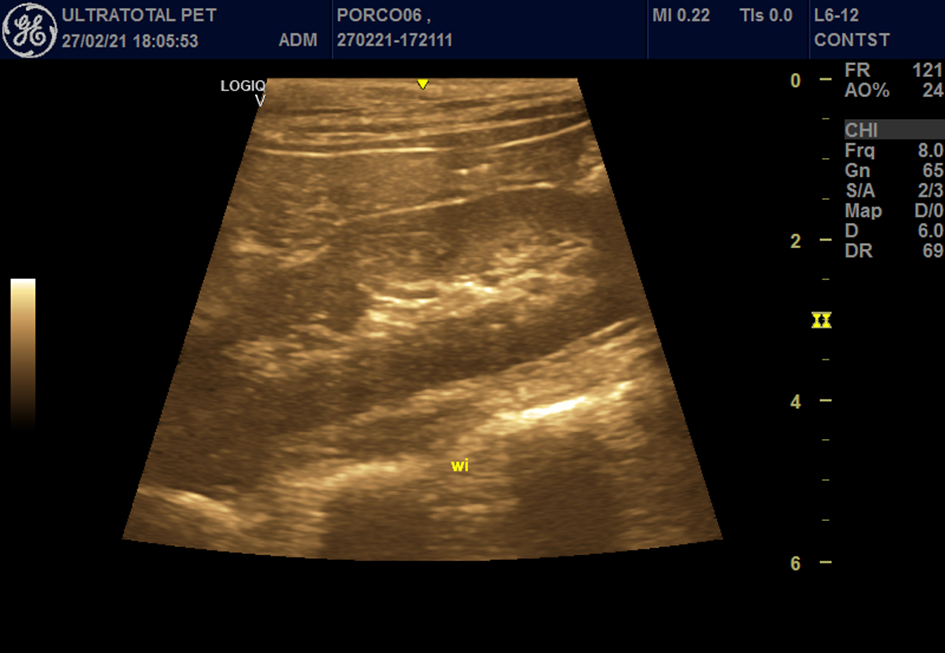
Figure 2 – Right kidney at the time of peak enhancement, after microbubble contrast administration.
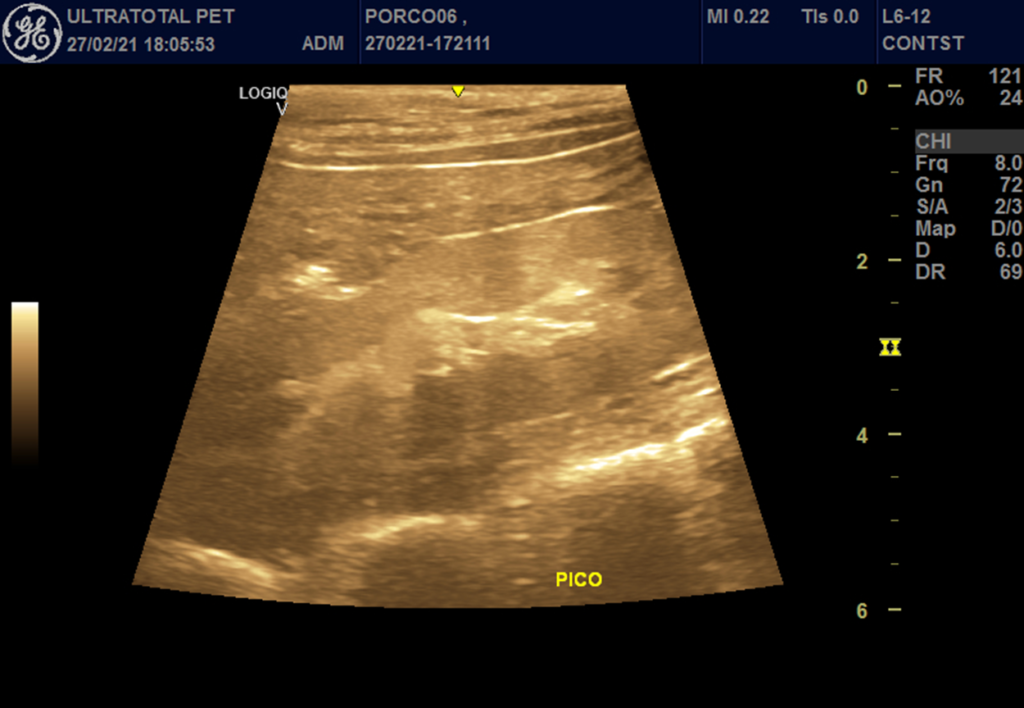
Figure 3 – Right kidney at the wash out moment, after microbubble contrast administration.
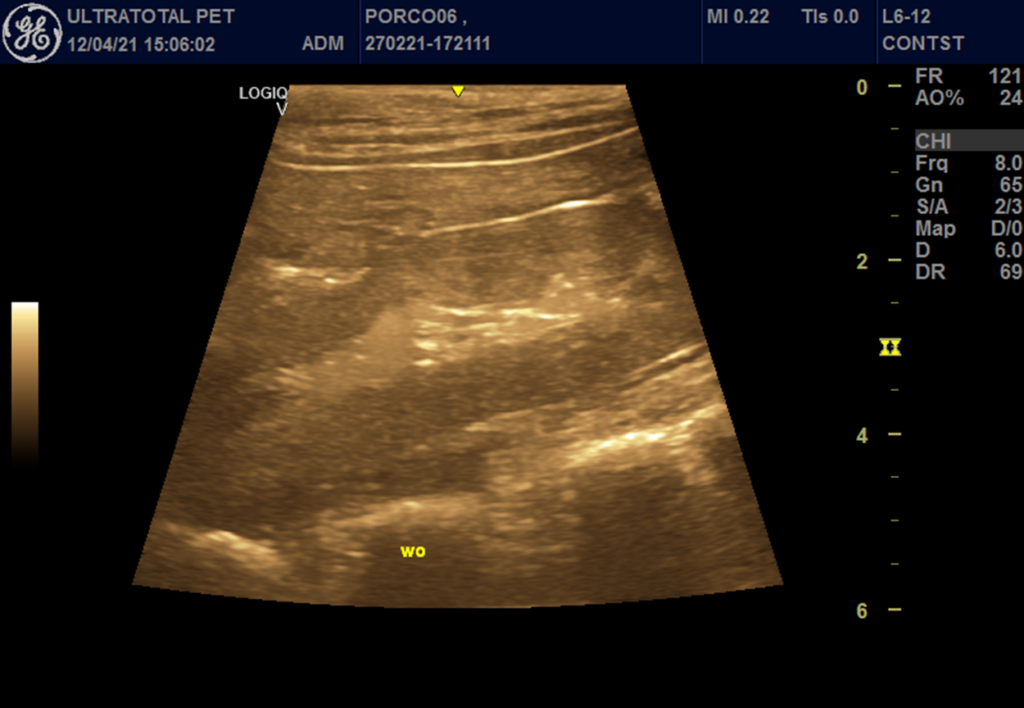
Figure 4 – Pre-operative (before) and post-operative (after) wash-in of pigs undergoing Inflow occlusion.
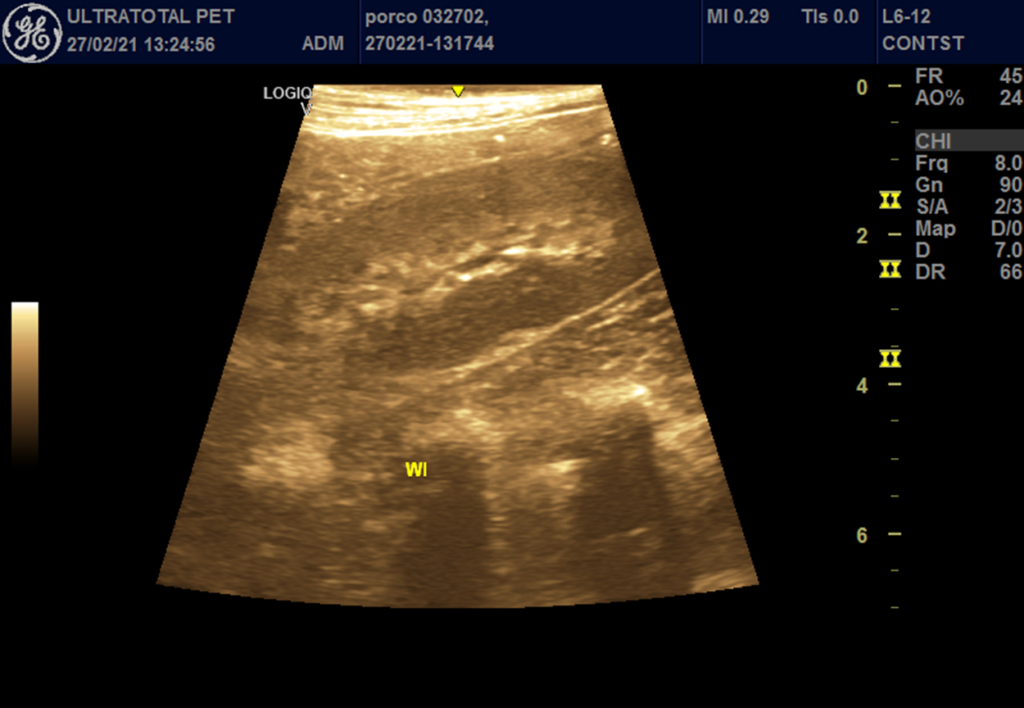
Pre-operative wash-in
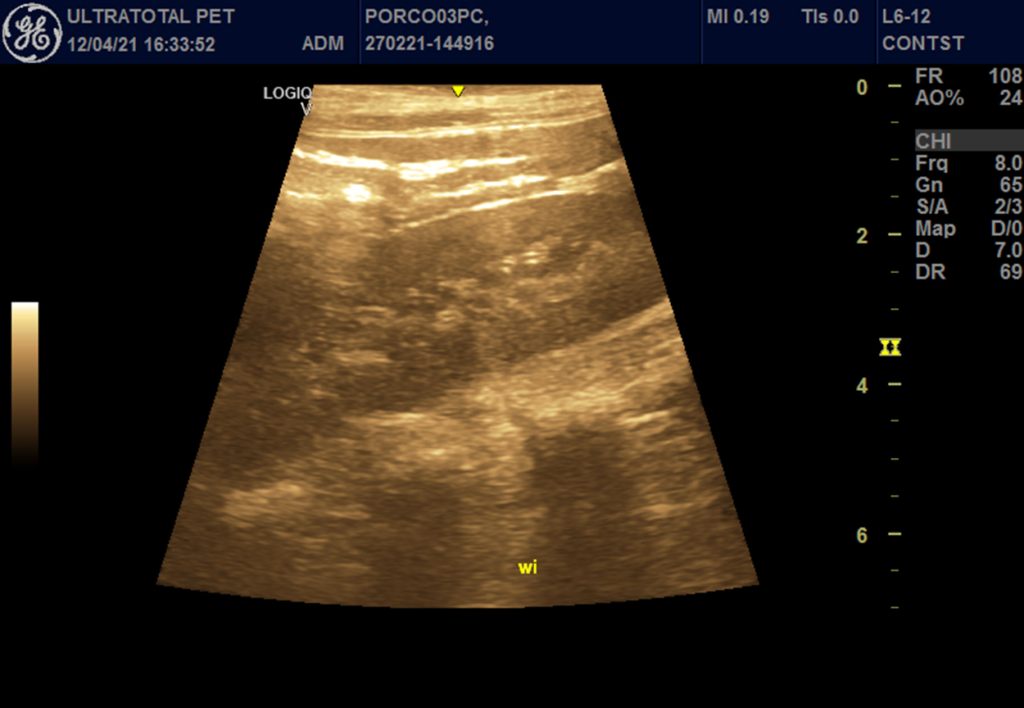
Post-operative wash-in
1Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0001-9265-2433
2Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0002-9914-9168
3Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0001-7849-9188
4Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0003-3914-1815
5Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0001-6596-8918
6São Paulo State University Julio Mesquita Filho, Department of Pathology, Reproduction and Health, Access Route Prof. Paulo Donato Castellane, CEP 14884-900, Jaboticabal, São Paulo, Brazil. ORCID: https://orcid.org/0000-0002-5517-4644
7Federal University of Santa Maria, Department of Large Animal Clinical Studies, Av. Roraima, 1000, Camobi, CEP: 97105-900, Santa Maria, Rio Grande do Sul, Brazil. ORCID: https://orcid.org/0000-0003-4373-6539
8Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, BrazilORCID: https://orcid.org/0000-0001-6257-9378
9Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0002-8601-6489
10Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/my-orcid
11 ORCID: https://orcid.org/0000-0001-6797-5388
12Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0002-2373-2453
13Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil ORCID: https://orcid.org/0000-0002-2885-9322
14 Author for correspondence: Darcy Ribeiro Norte Fluminense State University, High Complexity Surgery in Small Animals Sector of the Animal Experimentation Unit, Av. Alberto Lamego, 2000, CEP: 28013-602, Campos dos Goytacazes, Rio de Janeiro, Brazil. e-mail: lacerdavet@uol.com.br. https://orcid.org/0000-0003-0530-0785
