REGISTRO DOI: 10.5281/zenodo.7785746
Bianca Leite Magalhães¹
Abstract:
Introduction: The use of new technologies in healthcare has considerably increased the prognosis of patients in medical intensive care. Muscle weakness acquired in the intensive care unit (ICU) is a neuromuscular complication that affects between 30% and 60% of patients hospitalized in hospital settings. NMES is used to enhance muscle strengthening in individuals who have exercise intolerance or who are unable to perform active movements. Objectives: analyze the Neuromuscular Electrical Stimulation benefits and parameters used in patients in the ICU. Methods: This qualitative review article’s purpose is to analyze if NMES in the recovery of patients in the ICU is effective. For the study, some steps were followed, such as the establishment of the hypothesis, question, problem, choice of criteria for inclusion and exclusion of articles, the definition of information to be extracted, analysis, and discussion. Results: In total 16968 articles were found in the following databases: SciELO Brasil, PubMed, and ScienceDirect. After reading titles and abstracts 10 articles were selected to compose results and discussion. Conclusions: The disuse of muscles during the hospitalization period can lead to weakness and loss of muscle mass. NMES is a safe, low-cost, and easy-to-replicate alternative to prevent ICU-acquired weakness.
Keywords: Neuromuscular electrical stimulation. Acquired weaknesses. Critical patients, Physiotherapy
Introduction
The use of new technologies in healthcare has considerably increased the prognosis of patients in medical intensive care. It also can help to reduce the average number of days that patients spend in the hospital. Recent studies show that in 7 days of rest, muscle strength is reduced by 30%, followed by 20% weekly. About 20% to 25% of patients who need mechanical ventilation have problems weaning the ventilator due to musculoskeletal complications caused by immobility (Sachetti et al., 2017). ICU-acquired weaknesses can cause an increase in Intensive Care Unit costs due to prolonged hospital stays (Vanhorebeek, 2020).
During hospitalization, in addition to mechanical ventilation, patients usually need neuromuscular blockers that impair nerve impulse conduction, leading to muscle relaxation, which can cause muscle weakness, and the joints can become stiff (Tezcan, et al., 2020). Under these circumstances, the initial condition can worsen leading to ICU- acquired weaknesses. Sarcopenia is defined as the loss of muscle mass. One of the most common causes of sarcopenia is aging, but this condition can occur in patients with chronic systemic diseases, especially in hospitalized patients. Individuals with sarcopenia may face outcomes such as falls, loss of functionality, and mortality. The topic has become the focus of numerous research related to its pathophysiology in order to offer better treatments for patients to reduce the damage related to the condition (Cruz-Jentoft et al., 2019).
Although sarcopenia is more common in elderly people, hospitalized patients can present a secondary form of the condition due to muscle inactivity. UCI- acquired weaknesses may be considered one of the causes of secondary sarcopenia
(Kizilarslanoglu, 2016). According to Levy (2022), the prevalence of sarcopenia was more frequent in older females, presenting severe loss of mass after three months. With the high occupation of hospital beds due to the COVID-19 pandemic, more studies are needed to prove the effectiveness of therapies that help delay the loss of muscle mass, leading the patient to prolong hospitalization time. Neuromuscular stimulation is a tool used to increase muscle strength and prevent atrophy.
Muscle weakness acquired in the intensive care unit (ICU) is a neuromuscular complication that affects between 30% and 60% of patients hospitalized. The most common risk factors that lead to muscle weakness include systemic inflammatory response, the use of sedatives and neuromuscular blockers, hyperosmolarity, parenteral nutrition, and prolonged immobility (Miranda et al., 2013).
The time of immobilization in bed, and the systemic factors associated with the length of hospital stay, contribute to the prolonged stay of critical patients in the ICU. These complications usually interfere directly with the decline in functional status and quality of life and may persist with negative symptoms even after hospital discharge, which increases hospitalization costs in the post-discharge period (Miranda et al., 2013).
Martin et al., (2005) state that muscle weakness in critically ill patients is diffuse and symmetrical, affecting the peripheral and central muscles. In addition, Critical Patient Polyneuropathy, which has a high incidence in intensive care units (ICU), generates neural impairment that contributes to prolonging rehabilitation even after hospital discharge.
Many studies are unanimous in affirming the positive effects of early mobilization in the treatment of critically ill patients, however for better results and effective gains in functional independence, patient cooperation is necessary, a factor that is limited when the patients are receiving sedatives and analgesics (Gerovasili et al., 2009).
In order to minimize the harmful effects of hospitalization, Physiotherapy emerges in addition to new technologies, as a profession that aims to establish techniques, resources, and complete clinical care to prevent and treat these changes and promote health and quality of life in the hospital setting. Among these resources, electrical stimulation has been widely discussed as a beneficial coadjuvant factor in the treatment of these patients.
NMES is used to enhance muscle strength in individuals with exercise intolerance or who are unable to perform active movements. NMES with medium frequency currents, when compared to active movement, can activate 30% to 40% more motor units. This technique promotes alpha motor nerve modulation while active movement depolarizes the neuron. In addition, the electrical impulse initially recruits type II fibers, inversely to the active movement (Oliveira et al., 2009).
In this context, NMES can bring benefits to hospitalized patients through electrical impulses produced by a machine and supplied via electrodes placed close to the muscles that need to be stimulated, generating an electrical current that may be able to increase strength, muscle resistance, and also it can help to improve the patient’s overall condition (Reidel, 2020).
Methods
This descriptive bibliographic research aims to group and summarize scientific knowledge, helping to qualify clinical practice, using experimental and non-experimental studies based on empirical and theoretical knowledge (Alcoforado et al., 2014).
For the study, some steps were followed such as establishing the research’s guiding question, defining a hypothesis and specific objectives; data collection from the inclusion and exclusion criteria; categorization; critical reading of eligible articles, analysis of results, and synthesis of knowledge disclosed by the authors.
The guiding question of the research was: “is neurostimulation effective in the treatment of ICU patients?” National databases Scientific Electronic Library Online (SciELO Brasil), Science Direct, and National Library of Medicine (PubMed) were consulted. In order to guide the search, the following descriptors were used: eletroestimulação neuromuscular, neuromuscular electrical stimulation, sarcopenia, acquired weaknesses, ICU, critical patients, physiotherapy, and their combinations.
The objective of this review is to analyze whether NMES is effective in the recovery of critically ill hospitalized patients. Inclusion criteria were complete national or international articles in Portuguese, English, and Spanish, which addressed the topic in original research or literature reviews. The exclusion criteria for the articles were: repeated articles and NMES in non-hospitalized patients. After applying the criteria, six scientific articles were selected to compose this review study. The recommendations proposed in the PRISMA guide (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) were used, using its flowchart to organize the captured results, as shown in figure 1 (Alcoforado et al., 2014).
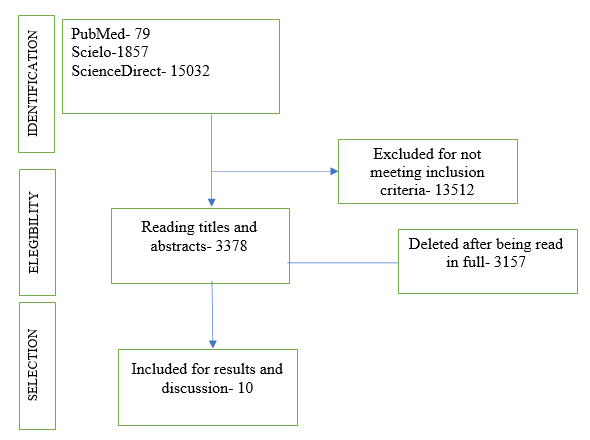
Results
The following table presents a summary of the results found after applying the article selection criteria. Despite being a safe and easy-to-apply resource, electrical muscle stimulation is still a topic not often discussed in the treatment of critically ill patients. There was a significant loss of studies because the intersection of keywords did not bring the research question closer to the eligibility criteria.
Therefore, the sample consisted of 6 articles that dealt with muscle electrostimulation as a therapeutic resource in the treatment of acquired muscle weakness in critical patients.
Table 1. Bibliographic approach on ENMS in critically ill patients.
Author Objective Methodology Results SILVA et al.,2017. Assess the safety and feasibility of NMES based on neuromuscular excitability. Observational study. NMES was applied for 15 minutes daily for 3 consecutive days, bilaterally in the following muscles: gluteus maximus, hamstrings, quadriceps femoris, gastrocnemius, and tibialis anterior. The pulse frequency used was 100 Hz, with an ON time of 5 seconds, an OFF time of 5 seconds, and no rise time. NMES is safe when applied in 5 muscle groups of critically ill patients. There was a difference in neuromuscular excitability among the muscles and patients tested, demonstrating the need for a customized protocol based on chronaxie. RIGHETTI et al., 2022. Analyze the effects of NMES on muscle mass and functionality of patients with severe COVID-19 associated with sepsis and septic shock. NMES was applied after the interruption of the neuromuscular blocker, seven patients received the treatment in the supine position and with 30-60 degrees of the hips and knees flexion. Two electrodes were positioned on the motor area of the vastus medialis and vastus lateralis muscles. The other two were placed on the inguinal region. The parameters were: stimulation frequency of 100 Hz, a stimulation pulse width of 350 μs, a ramp-up time of 1 s, time on of 4 s, a rampdow of the stimulation of the 1 s, and time off of 12 s. Five patients completed the NMES protocol (1 died, and 1 stopped the treatment). The remaining patients had an increase in the MRC score and a decrease in the ICF-muscle strength, indicating an improvement in muscle strength. NONOYAMA, et al., 2022. The objective of this study was to analyze the benefits of NMES in older patients admitted to the ICU. The study divided older patients (≥65 years old) into the NMES group (n= 22) and control group (n= 22). The following settings were applied: frequency of 20 Hz, a pulse width of 250 μs, and a duty cycle of 5 seconds of stimulation followed by 2 seconds of pause after 48 hours of admission, 30 minutes daily, 5 days/week. Muscle thickness was present in the control group. The NMES group had an inferior muscle thickness reduction. There was not a significant difference in physical functioning between the two groups. KHO et al., 2013. Describe a protocol for a randomized, phase II pilot study of NMES in patients receiving mechanical ventilation, and summarize recent studies of early rehabilitation and
NMES in the ICU.Randomized, sham-controlled, concealed, phase II pilot study. Patients received NMES applied to the quadriceps, tibialis anterior, and
gastrocnemius muscles for 60 minutes per day.NMES can improve by 25% of muscle strength. Early intervention may have an important impact on short and long- terms outcomes. SEGERS et al.,
2014Investigate the safety and feasibility of NMES in critically ill patients. Observational study.
50 patients hospitalized for at least 6 days were included on days 3 to 5. Patients with neuromuscular disorders and patients with musculoskeletal conditions limiting quadriceps contraction were excluded. NMES was applied for 25 minutes, 5 days/ week on the quadriceps.NMES is safe to be used in ICU. Quadriceps contraction was less likely in patients presenting the following conditions: sepsis, edema, and receiving vasopressors. KUMAR et al., 2022 Assess the impact of NMES in improving dyspnea in Post- COVID syndrome patients. Multicentric randomized shamcontrolled, doubleblinded parallel phase 2 superiority trial. NMES was used after physical therapy sessions for 30 minutes, 3 times a week, for 90 days in the interventional group. The electrodes were applied to the quadriceps muscles. The intensity was adjusted according to the patient’s sensibility. A biphasic pulse of 50 Hz and a pulse duration of 400 ms was used. The blind group received the traditional PT treatment and 30 minutes of sham
NMES. Patients were evaluated for dyspnea through a 6-minute walk test. NMES is low-cost, easy to replicate, and nonpharmacological that can be used as a
complementary technique that can help to improve PCS symptoms.
Discussion
ICU- acquired weaknesses are a consequence of the interaction of bed rest and muscle loss, its pathophysiology is characterized by a decrease in muscle protein synthesis, thus increasing the process of muscle catabolism, causing loss of muscle strength and mass. ICUAW is present in more than 50% of hospitalized patients who need mechanical ventilation for more than 48 hours. Some factors as the use of the prolonged use of neuromuscular blockers, sepsis and multi-organ failure, hyperglycemia, and the use of corticosteroids can contribute to the condition. The duration of bed rest, hospital stay, and ventilation can also cause ICU-acquired weakness (Hodson, 2017). This condition can be developed due to a variety of etiologies, including trauma, surgery, and acute illness. Muscle loss is associated with changes in microvascular, electrical, and metabolic mechanisms and bioenergetic changes, leading to loss of muscle strength and, consequently, atrophy. ICUAW is tested in cooperative patients through the Medical Research Council Score. MRC establishes muscle strength through a score between 0 (no contraction) and 5 (full contraction), being evaluated among the 12 muscle groups. The sum score is between 0 to 60. Patients with a score < 48 are diagnosed with ICUAW (Hermans, 2015).
Table 2. Medical Research Council Score
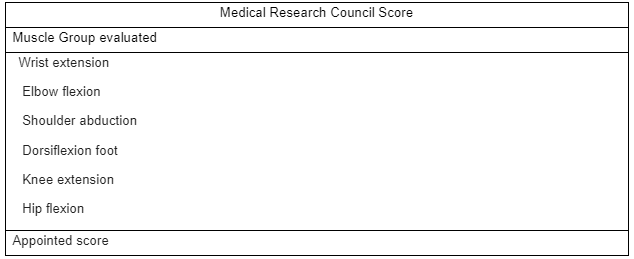
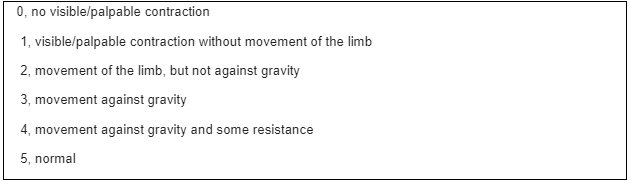
Source: Adapted from Hermans, 2015
The impact on the quality of life of these patients is severe, even after hospital discharge. In this context, neuromuscular electrical stimulation is being used as an involuntary contraction in the muscle when the voluntary contraction is impaired. Among the advantages of the technique, studies cite increased muscle strength, prevention of atrophy, improved muscle function, maintenance of blood flow, and reduced edema. Studies demonstrate that NMES parameters are normally applied at a frequency between 30-40Hz, and a pulse duration of 250–400 μsis adjusted according to the patient’s tolerance. The contraction must need to be visible. Most protocols use NMES for 60 minutes or two sessions of 30 minutes daily (Burgess L. et al., 2021). When neuromuscular electrostimulation is applied in the early stages of hospitalization, it can help patients to enhance their capabilities due to can improve muscle strength, and reduce the duration of mechanical ventilation, leading to a decrease in the length of the ICU stay (Liu, 2020).
SILVA P, et al., 2017 evaluated the safety and feasibility of NMES based on neuromuscular excitability and observed that NMES is safe when applied to critically ill patients. The authors also concluded that there is a difference in neuromuscular excitability between the muscles in patients tested, demonstrating the need for a customized protocol based on chronaxie, the minimum time for excitation of a structure that causes a muscle response.
Still, with regard to feasibility and safety, SEGERS J, et al., 2014, stated that NMES is safe to be used in the ICU. In this study, it was possible to observe that quadriceps contraction was less likely in patients with sepsis, edema, and the use of vasopressors.
In 2013, KHO M, et al., through a randomized pilot study, had already described a phase II randomized protocol for the application of NMES in patients receiving mechanical ventilation and the impact of early rehabilitation and NMES in the ICU. These patients received electrical stimulation in previously established muscle groups. Corroborating with Silva, et al., they confirmed that the use of EMNS improves muscle strength by 25% and that early intervention can have an important impact on short- and long-term results. The COVID-19 pandemic has brought several complications to the population, including a large number of morbidity and mortality due to complications associated with extended hospitalization. KUMAR A, et al., 2022, evaluated the impact of NMES on the improvement of dyspnea in patients with post-COVID syndrome (PCS) and observed that electrical stimulation proved to be effective and safe to use as a complementary technique promoting the improvement of symptoms of Post-COVID conditions.
Conclusion
Many of the patients who underwent hospitalization had not only respiratory but also physical impairments. Weakness and loss of muscle mass are often seen in these patients. Studies are being carried out to demonstrate the effectiveness of various treatments to restore functional capacity after discharge. Patients who have ICU-acquired muscle weakness describe the impact of the NMES resource on increased muscle strength, functional independence, decreased hospitalization time, mechanical ventilation time, and enhanced recovery. NMES proved to be a safe and low-cost intervention that does not have adverse effects and may be a promising approach for rehabilitation. NMES can provide increased muscle strength, increased functional capacity, and a significant decrease in recovery time. However, more studies must be conducted to analyze if the technique can help decrease the length of hospital stay and the time of need for invasive mechanical ventilation.
References
ALCOFORADO, C, L, G, C.; ERCOLE, F, F.; MELO, L, S.; Revisão integrativa versus revisão sistemática; Revista mineira de enfermagem. v18, 2014.
Burgess, L. C., Venugopalan, L., Badger, J., Street, T., Gad, A. L. O. N., Jarvis, J. C., & Swain, I. D. (2021). Effect of neuromuscular electrical stimulation on the recovery of people with COVID-19 admitted to the intensive care unit: a narrative review. Journal of rehabilitation medicine, 53(3).
Cruz-Jentoft, A. J., Baeyens, J. P., Bauer, J. M., Boirie, Y., Cederholm, T., Landi, F., Martin, F. C., Michel, J. P., Rolland, Y., Schneider, S. M., Topinková, E., Vandewoude, M., Zamboni, M., & European Working Group on Sarcopenia in Older People (2010).
Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing, 39(4), 412–423. https://doi.org/10.1093/ageing/afq034
Levy, D., Giannini, M., Oulehri, W., Riou, M., Marcot, C., Pizzimenti, M., Debrut, L., Charloux, A., Geny, B., & Meyer, A. (2022). Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients, 14(4), 912. https://doi.org/10.3390/nu14040912
Gerovasili, Vasiliki, et al. “Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study.” Critical care 13 (2009): 1-8.
Hermans, G., & Van den Berghe, G. (2015). Clinical review: intensive care unit acquired weakness. Critical care (London, England), 19(1), 274. https://doi.org/10.1186/s13054- 015-0993-7
Hodgson, C. L., & Tipping, C. J. (2017). Physiotherapy management of intensive care unit-acquired weakness. Journal of Physiotherapy, 63(1), 4-10. https://doi.org/10.1016/j.jphys.2016.10.011
Kizilarslanoglu, M. C., Kuyumcu, M. E., Yesil, Y., & Halil, M. (2016). Sarcopenia in critically ill patients. Journal of anesthesia, 30(5), 884–890. https://doi.org/10.1007/s00540-016-2211-4
Kho, M. E., Truong, A. D., Brower, R. G., Palmer, J. B., Fan, E., Zanni, J. M., Ciesla, N. D., Feldman, D. R., Korupolu, R., & Needham, D. M. (2012). Neuromuscular Electrical, Stimulation for Intensive Care Unit-Acquired Weakness: Protocol and
Methodological Implications for a Randomized, Sham-Controlled, Phase II Trial. Physical Therapy, 92(12), 1564-79. https://doi.org/10.2522/ptj.20110437
Kumar, A. A., Xicota, C. N., Chin, A. C., Pelanda, J. D. C., Monaco, T. D. O., Mohamed,
L. A. A., … & Sukik, A. A. M. (2022). Neuromuscular Electrical Stimulation (NMES) as an Add-on Therapy for the Improvement of Dyspnea in Patients with Post-Covid Syndrome: a Protocol for a Phase II Randomized, Non-Pharmacological Intervention- Controlled, Double-Blind Study. Principles and Practice of Clinical Research, 8(3), 19- 28.
Liu, M., Luo, J., Zhou, J., & Zhu, X. (2020). Intervention effect of neuromuscular electrical stimulation on ICU acquired weakness: a meta-analysis. International Journal of Nursing Sciences, 7(2), 228-237.
Miranda et al., Eletroestimulação em doentes críticos: uma revisão sistemática. Revista Pesquisa em Fisioterapia, Salvador, 2013 Jul;3(1): 79-91. http://www.bahiana.edu.br/revistas.
Nonoyama, T., Shigemi, H., Kubota, M., Matsumine, A., Shigemi, K., & Ishizuka, T. (2022). Neuromuscular electrical stimulation in the intensive care unit prevents muscle atrophy in critically ill older patients: A retrospective cohort study. Medicine, 101(31), e29451. https://doi.org/10.1097/MD.0000000000029451
Oliveira, Danathielle Atique Rei de; et al. Fortalecimento da Musculatura Respiratória Através da Corrente Russa em Paciente com Doença Pulmonar Obstrutiva Crônica – Relato de Caso. Rev. Eletrônica Interfisio, 2009.
Segers, J., Hermans, G., Bruyninckx, F., Meyfroidt, G., Langer, D., & Gosselink, R. (2014). Feasibility of neuromuscular electrical stimulation in critically ill patients. Journal of critical care, 29(6), 1082-1088.
Reidel, L. T., Cecchele, B., Sachetti, A., & Calegari, L. (2020). Effects of neuromuscular electrostimulation of quadriceps on the functionality of fragileand pre-frail hospitalized older adults: randomized clinical trial. Fisioterapia e Pesquisa, 27, 126-132.
Righetti, R. F., Grams, S. T., Costa, W. N. D. S., Saraiva, L. T., de Salles, I. C. D., & Yamaguti, W. P. (2022). Neuromuscular Electrical Stimulation in Patients With Severe COVID-19 Associated With Sepsis and Septic Shock. Frontiers in medicine, 9, 751636. https://doi.org/10.3389/fmed.2022.751636
Sachetti, A., Dal’Acqua, A. M., de Aguiar Lemos, F., da Silva Naue, W., dos Santos, L. J., Bianchi, T., & Dias, A. S. (2017). Efeitos da estimulação elétrica neuromuscular sobre a mobilidade diafragmática de pacientes críticos: ensaio clínico randomizado. ConScientiae Saúde, 16(2), 224-233.
Silva, P. E., Babault, N., Mazullo, J. B., de Oliveira, T. P., Lemos, B. L., Carvalho, V. O., & Durigan, J. L. Q. (2017). Safety and feasibility of a neuromuscular electrical stimulation chronaxie-based protocol in critical ill patients: A prospective observational study. Journal of critical care, 37, 141–148. https://doi.org/10.1016/j.jcrc.2016.09.012 SOUSA, E. F. Efeitos da Eletroestimulação Neuromuscular em pacientes críticos: uma revisão de literatura. 2016. 49f. Tese (Aprimoramento) – Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, 2016.
Tezcan, B., Turan, S., & Özgök, A. (2019). Current Use of Neuromuscular Blocking Agents in Intensive Care Units. Turkish journal of anaesthesiology and reanimation, 47(4), 273–281. https://doi.org/10.5152/TJAR.2019.33269
Vanhorebeek, I., Latronico, N., & Van den Berghe, G. (2020). ICU-acquired weakness. Intensive care medicine, 46(4), 637–653. https://doi.org/10.1007/s00134- 020-05944-4.
1Graduated Physical therapist from FACIMA (2014) and a Master’s degree in Business earned from Schiller International University (2023). Developed my activities in a non- profit association treating children with different neurological conditions. Provided home care services and treatment to patients with the most diverse types of diagnoses and needs in trauma-orthopedics, neurology, and pediatrics fields.
