SOBREVIVÊNCIA E ALTERAÇÕES NO NÍVEL ÓSSEO MARGINAL DE IMPLANTES CERÂMICOS INJETADOS DE DUAS PEÇAS: ESTUDO CLÍNICO RETROSPECTIVO
REGISTRO DOI: 10.69849/revistaft/cl10202411301120
Gustavo Moreno Braga, DDS, MSc
Marcelo Noboru Tanizaka, DDS, MSc
Carolina Accorsi Cartelli, DDS, MSc
Erton Massamitsu Miyasawa, DDS, MSc, PhD
Flávia Noemy Gasparini Kiatake Fontão, DDS, MSc, PhD
Geninho Thomé, DDS, MSc, PhD
Luis Eduardo Marques Padovan, DDS, MSc, PhD
RESUMO
A reabilitação de pacientes totalmente ou parcialmente edêntulos com implantes dentários é um tratamento amplamente consolidado na implantodontia. No entanto, desvantagens associadas aos implantes de titânio, como hipersensibilidade e possíveis comprometimentos estéticos em áreas com perfis gengivais delgados, têm incentivado o desenvolvimento de implantes cerâmicos. O objetivo deste estudo foi avaliar retrospectivamente a taxa de sobrevivência e as alterações no nível ósseo marginal de implantes cerâmicos usinados por injeção em duas peças (Sistema de Implantes Cerâmicos Zi Neodent®) após até 18 meses da instalação. A amostra foi composta por 23 pacientes, totalizando 27 implantes cerâmicos instalados. Os dados foram coletados dos prontuários clínicos, e as alterações no nível ósseo foram analisadas por dois avaliadores experientes e previamente calibrados, utilizando radiografias periapicais digitais. As taxas de sobrevivência dos implantes e das próteses foram de 100%, observadas em um período médio de 16,2 ± 9,8 meses após a instalação dos implantes. A média de alteração no nível ósseo marginal foi de 1,07 ± 0,07 mm durante o período avaliado. Conclui-se, portanto, que os implantes cerâmicos representam uma alternativa viável e previsível aos implantes de titânio, apresentando alta taxa de sobrevivência e excelente manutenção do nível ósseo em até 18 meses de pós-operatório.
Palavras-chave: implantes dentários, zircônia tetragonal estabilizada por ítria, taxa de sobrevivência.
ABSTRACT
The rehabilitation of fully and partially edentulous patients with dental implants is a well-established treatment in implant dentistry. However, disadvantages associated with titanium, such as hypersensitivity and possible impairment in esthetic areas with thin gingival profiles, have prompted the development of ceramic implants. The objective of this study was to retrospectively evaluate the survival rate and marginal bone level changes of two-piece injection-molded ceramic implants (Neodent® Zi Ceramic Implant System) after up to 18 months of implant placement. The sample comprised 23 patients, with a total of 27 ceramic implants placed. Data were collected from patient records and bone level changes analyzed by two experienced and previously calibrated evaluators using digital periapical radiographs. The survival rates for implants and prostheses were both 100%, observed over a mean of 16.2 ± 9.8 months following implant placement. Mean marginal bone level change was 1.07 ± 0.07 during the evaluated period. Therefore, it was concluded that ceramic implants are a suitable and predictable alternative to titanium implants, whereas they present high survival rate and excellent bone level maintenance in up to 18 months post-surgery.
Keywords: dental implants, yttria stabilized tetragonal zirconia, survival rate.
INTRODUCTION
Dental implants provide predictable and well-established treatment in implant dentistry, rehabilitating fully and partially edentulous patients. They present high biocompatibility and offer diverse treatment possibilities. However, in recent years, significant issues have come to light, such as titanium hypersensitivity. A clinical study revealed through MELISA testing (Memory Lymphocyte Stimulation Assay) that titanium can induce clinically significant hypersensitivity in patients chronically exposed via dental implants, even if they tested negative for titanium allergy through patch testing [1]. Another factor to consider is the corrosion of this material, with investigations showing an increase in titanium particle concentration in the bone tissue adjacent to dental implants and in regional lymph nodes [2,3]. Moreover, in esthetically demanding areas with thin gingival biotype or compromised soft tissues, the grayish color of titanium implants may be unfavorable and challenging, compromising aesthetics [4,5]. As a result, ceramic implants have attracted considerable attention in recent studies.
Among ceramic materials, yttria-stabilized tetragonal zirconia polycrystals (YTZP) have been chosen as the material of choice for manufacturing ceramic implants. YTZP exhibits high flexural strength, fracture toughness, corrosion resistance, and wear resistance [6,7,8]. Moreover, they present excellent esthetics due to their whitish color, are chemically inert with minimal adverse reactions, promote good cellular adhesion, excellent tissue response, and high biocompatibility [9].
More recently, the injection molding technique has been introduced for the manufacturing of ceramic implants. It offers additional advantages such as osseointegration equivalent to that of titanium implants, while significantly higher to that of machined ceramic implants, in addition to the ability to manufacture and design the implant surface in a single process, directly incorporated into the mold [10,11,12].
As for two-piece ceramic dental implants, they provide advantages over single-piece implants, such as greater prosthetic versatility, avoidance of problems during the healing period related to unwanted loads, and possibility of simultaneous bone augmentation procedures [13]. However, clinical studies are necessary for a better long-term evaluation.
Thus, this retrospective study aimed to evaluate the implant and prosthesis survival rates, as well as marginal bone level changes around two-piece injection-molded ceramic implants over a minimum of 12 months after placement.
MATERIALS AND METHODS
After approval by the Research Ethical Committee, the sample was retrospectively selected and based on the pool of patients who had two-piece injection-molded ceramic implants (Zi Ceramic Implant System, Neodent, Curitiba, Brazil) placed at Ilapeo College, between 2019 and 2022.
Patients aged 18 years and older were included if their dental records included data from at least one year of post-surgical clinical follow-up. Additional inclusion criteria involved radiographs taken using the parallel technique, with correct positioning assessed based on clear visualization of the implant threads and cervical region, both at the time of implant placement and at least 1 year thereafter.
Exclusion criteria were planned based on potential confounding factors that could compromise the validity of radiographic assessments. Patients were therefore excluded if they had systemic conditions such as poorly controlled diabetes, hypoparathyroidism, drug or alcohol dependence, chronic steroid or bisphosphonate use, or a history of radiation therapy to the head and neck region within the past 5 years. Also excluded were patients with heavy smoking (10 or more cigarettes per day), significant occlusal alterations potentially impacting implant stability or function, a history of aggressive periodontal disease or generalized chronic periodontitis influencing peri-implant soft tissue health.
Patient records were accessed to collect demographic data (sex, age), history of systemic diseases, surgery date, implant region, implant characteristics (dimensions, final insertion torque), loading protocol (“immediate” meaning in up to 7 days and “delayed” after 4 to 6 months following implant placement), and data on intra- and post-operative complications. Periapical radiographs were additionally retrieved for the following time points: immediately after placement (T0), 6 months (T1), 12 months (T2), and 18 months (T3) after placement.
To evaluate marginal bone height, Sidexis XG software version 4.0 (Sirona, Bensheim, Germany) was used to perform vertical linear measurements on the mesial and distal surfaces of the implants. Initially, calibration of the image size was required based on the actual implant diameter measurement. For this purpose, a horizontal linear measurement was performed on the implant platform using a calibration tool, and this value was adjusted according to the real diameter measurement of the implant (Fig. 1).
Fig. 1 – Calibration of the radiographic image based on the actual measurement of the implant diameter
After software calibration, horizontal reference lines were drawn. The first reference line tangentially located at the implant platform that was evaluated (Fig. 2). The next reference line located at the first bone contact point of the cervical margin of the implant, considering the mesial and distal surfaces (Fig. 3). Thus, it was possible to evaluate the vertical bone level around the zirconia implants, which could show a positive value when the bone-implant contact was located above the implant platform (Fig. 4a), and a negative value when it was below the implant platform (Fig. 4b). Mesial and distal bone levels of the implant were averaged to determine the final bone level. Measurements were obtained by two experienced operators previously calibrated following the described standardized method for all time points. An assessment of inter-examiner error was conducted to ensure consistency between the two operators.
Fig. 2 – Horizontal reference line (red) tangent to the implant platform
Fig. 3 – Horizontal reference line (blue) considering the first mesial (A) and distal (B) bone contact with the cervical margin of the implant
Fig. 4 – a) Positive bone level measurement (bone above the platform level); b) negative bone level measurement (bone below the platform level)
Bone level change was calculated as the mean difference between bone level at T3 and at baseline (T0). Implant and prosthesis survival were defined as no loss of the implant/prosthesis up to the last follow-up record.
Data Analysis
Summary descriptive statistics were calculated for all study parameters. Quantitative parameters were described using mean and standard deviation, while qualitative variables were presented as frequencies and percentages. Survival rates were calculated by dividing the number of events by the total number of implants evaluated. Normal distribution of data was verified using the Kolmogorov-Smirnov test. Since the variable showed a normal distribution, comparison between different study stages for “Bone Level” was performed using paired t-tests.
Results were considered significant for p<0.05. All analyses were conducted using Statistica software for Windows 10.0 (Statsoft, Tulsa, Okla). The methodology was reviewed by an independent statistician.
RESULTS
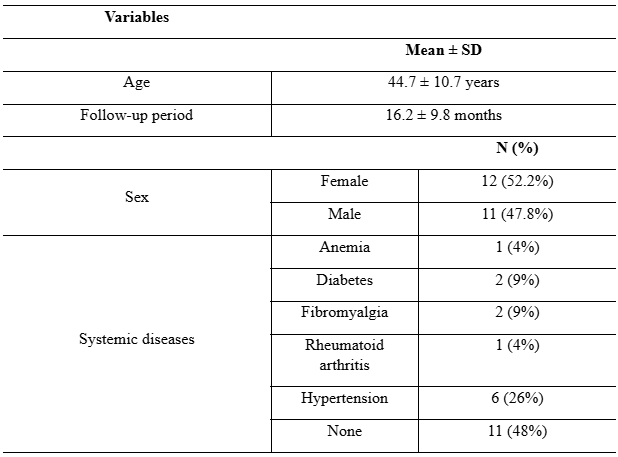
The sample comprised 23 patients, with a total of 27 ceramic implants (Neodent® Zi Ceramic Implant System) placed. The mean age of the subjects was 44.7 ± 10.7 years, with females being the most prevalent at 52.2%. Controlled hypertension was the most commonly recorded systemic disease (26%), while the majority of patients (48%) did not present any systemic disease (Table 1). Mean follow-up period was 16.2 ± 9.8 months after implant placement.
Table 1: Descriptive analysis of the variables evaluated at the patient level (n = 23)
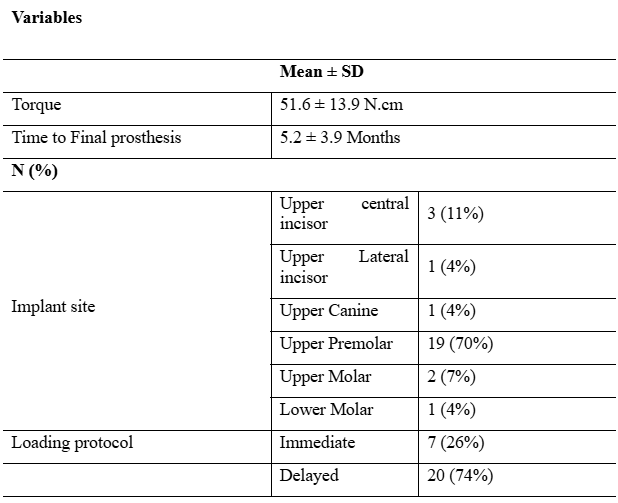
Mean insertion torque of the ceramic implants was 51.6 ± 13.9 N.cm. Time to installation of the final prosthesis was 5.2 ± 3.9 months. Upper first premolar showed the highest frequency for implant placement site (70%), followed by the upper central incisor (11%; Table 2). Xenogeneic bone grafts were in some cases needed, particularly after immediate placement following extractions, by means of filling the gap in the vestibular wall.
Table 2: Descriptive analysis of the variables evaluated at the implant level (n = 27)
Statistically significant differences (p=0.002, Table 3) were found in bone level among the different stages of evaluation. A significantly lower mean bone level was observed at T3 compared to baseline and T1, as well as between T2 and baseline. Mean marginal bone level change was 1.07 ± 0.07 during the evaluated period.
Table 3: Comparison among different study stages for Bone Level (n=27; repeated measures ANOVA)
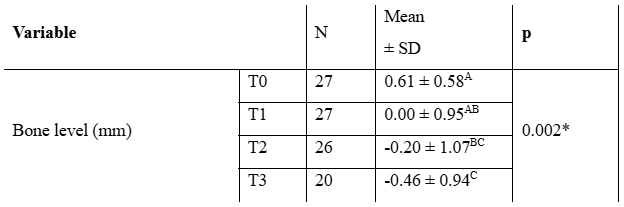
Note: *Statistically significant for p < 0.05. Different letters indicate statistically significant differences.
None of the study implants or prostheses were lost during the follow-up period, resulting in a 100% survival rate for both implants and prostheses.
DISCUSSION
The first ceramic dental implants were developed in the 1960s, according to Gahlert et al [14], and were made of aluminum oxide. However, their biomechanical properties, such as fracture toughness, were unsatisfactory, leading to fractures under axial loads, making them unsuitable for dental implants use. In the 1990s, a new ceramic material, zirconium dioxide, was introduced, presenting improved properties.
Gungor et al. [15] and Thome et al. [16] highlighted that in recent years that yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) has been chosen as the material of choice for manufacturing ceramic implants. Y-TZP consists of zirconium dioxide and yttrium oxide particles, forming a stable tetragonal structure at room temperature, capable of reducing crack propagation and providing high strength and toughness. In the present study, this was the raw material used to fabricate the analyzed implants, and its advantages were confirmed as no fractures occurred in the system even after immediate loading.
The manufacturing method for the study implants is injection molding, which, according to Thome et al.[16], is based on the plasticity of a zirconia formulation in the shape of the implant body. A minipig studies conducted by the same author demonstrated equivalence in osseointegration and bone formation around the implant, when compared to a titanium implant [17].
The survival rate of zirconia implants in the current study was 100% during an 18-month follow-up period. Mellinghoff [18] conducted a study involving 189 single-piece ceramic implants placed in 71 patients, achieving a survival rate of 93% after one year. Subsequently, Kohal et al. [19] investigated clinical and radiographic outcomes of one-piece ceramic implants, reporting a survival rate of 95.4% over the same period. Hashim et al. [20], in a systematic review and meta-analysis, found a survival rate of 92% for two-piece machined ceramic implants. Thus, our study has demonstrated superior outcomes for two-piece ceramic implants compared to the literature on one-piece and/or machined implants.
It is well known that ceramic implants exhibit osseointegration equivalent to titanium implants, which explains their high survival rate. Gungor et al. [15] emphasized that animal studies showed direct bone deposition on the zirconia surface, with good adhesion and proliferation of osteoblastic cells. Two systematic reviews included in the study by Cionca et al. [21] compared the osseointegration of zirconia implants to titanium implants. The parameters used were bone-implant contact and removal torque, showing no statistically significant difference between the two.
One-piece implants have some disadvantages, as described by Jank and Hochgatterer [13], such as the impossibility of using associated biomaterials, healing issues, and the potential for undesirable loads. Gungor et al. [15] stated that two-piece implants can minimize these problems. They are preferable when ideal primary stability is not achieved, can be used in conjunction with bone augmentation procedures, and minimize undesirable loads. As bone grafts were used in conjunction with the dental implants in the present study, cover screws were used for complete sealing, with occlusal loading at a later stage, thus taking advantage of the two-piece implant benefits.
The most common region for implant placement was the anterior maxilla and first premolars, which is ceramic implants primary indication. Gungor et al. [16] Emphasized that titanium implants can be visible through thin alveolar mucosa, compromising aesthetic results. Afrashtehfar and Del Fabbro [22] recommend zirconia implants for highly demanding aesthetic situations, involving the anterior maxilla and compromised soft tissues in this region.
When analyzing the marginal bone level over the different study stages, it was possible to observe that in 18 months of follow-up marginal bone level changes remained within the acceptable limits described in the literature of up to 1.5mm of bone remodeling after one-year of placement [23]. The study by Kohal et al. [19] found marginal bone loss of 1.31mm after one year of follow-up. Borgonovo et al. [23] evaluated radiographic bone loss around zirconia implants with single or multiple rehabilitations and found a mean 1.6mm after three years. In the study by Payer et al. [24], where single-piece zirconia implants were immediately loaded and rehabilitated, mean bone loss was found to be 1.3mm after 24 months. The bone level values observed in the present study suggest peri-implant bone maintenance should be expected in the long-term.
Although soft tissue aesthetics are important in assessing the cosmetic outcomes of dental implants, implant survival and bone loss evaluation remains crucial for the clinical and functional success of dental implant treatments. These assessments play a pivotal role in dental practice, even in the absence of specific data on soft tissue aesthetics. They are fundamental in determining the long-term clinical success of dental implants, directly influencing chewing function, occlusal stability, and overall oral health of patients. Besides serving as objective indicators of treatment efficacy, they aid in informed clinical decision-making, such as adjustments in implant loading and maintenance planning. Continuous research and development in this field are driven by data on implant survival and bone loss, contributing to advancements in implantation techniques, implant materials, and treatment protocols.
CONCLUSION
The two-piece injection molded ceramic implants (Neodent® Zi ceramic implant system) are a suitable and predictable alternative to titanium implants, whereas they present high survival rate and excellent bone level maintenance in up to 18 months post-surgery.
Acknowledgements: The authors would like to thank Waleska Furquim for her contribution as independent statistician.
DECLARATIONS
Ethics approval: The study was approved by Ilapeo College institutional research ethics committee and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent do Participate: Written consent was waived by the research ethics committee for the retrospective data collection involving the study participants. The confidentiality and anonymity of all participants were maintained throughout the research process.
Funding: No funding was received to assist with the preparation of this manuscript.
Availability of data and materials: Sequence data supporting the findings of this study are available upon request from the corresponding author.
REFERENCES
1. Muller K, Valentine-Thon E. Hypersensitivity to titanium: clinical and laboratory evidence. Neuro Endocrinol Lett 2006 Dec;27:31–35.
2. Bianco PD, Ducheyne P, Cuckler JM. Local accumulation of titanium released from a titanium implant in the absence of wear. J Biomed Mater Res. 1996 Jun;31(2):227–234.
3. Weingart D, Steinemann S, Schilli W, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994 Dec;23(6pt2):450–452.
4. Bosshardt DD, Chappuis V, Buser D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000 2017 Feb;73(1):22-40.
5. Van Brakel R, Noordmans HJ, Frenken J, de Roode R, de Wit GC, Cune MS. The effect of zirconia and titanium implant abutments on light reflection of the support. Clin Oral Implants Res. 2011 Oct;22(10):1172-78.
6. Hisbergues M, Vendeville S, Vendeville P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J Biomed Mater Res B Appl Biomater. 2009 Feb;88(2):519–29.
7. Kohal RJ, Wolkewitz M, Tsakona A. The effects of cyclic loading and preparation on the fracture strength of zirconium. Clin Oral Implants Res. 2011 Aug;22(8):808-14.
8. Osman RB, Swain MV. A critical review of dental implant materials with an emphasis on titanium versus zircônia. Materials (Basel). 2015 Mar;8(3)932-58.
9. Sivaraman K, Chopra A, Narayan AI, BalaKrishnan D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J Prosthodont Res. 2018 Apr;62(2):121-33.
10. Kim HK, Woo KM, Shon WJ, et al. Comparison of peri-implant bone formation around injection-molded and machined surface zirconia implants in rabbit tibiae. Dent Mater J. 2015 Sep;34(4):508- 515.
11. Thomé G, Sandgren R, Bernardes S, et al. Osseointegration of a novel injection molded 2-piece ceramic dental implant : a study in minipigs. Clin Oral Investig. 2021 Feb;25(2):603-15.
12. Lughi V, Sergo V. Low temperature degradation-aging-of zirconia: a critical review of the relevant aspects in dentistry. Dent Mater 2010 Aug;26(8):807-20.
13. Jank S, Hochgatterer G. Success Rate of Two-Piece Zirconia Implants: A Retrospective Statistical Analysis. Implant Dent. 2016 Apr;25(2):193-8.
14. Gahlert M, Röhling S, Wieland M, Sprecher CM, Kniha H, Milz S. Osseointegration of zirconia and titanium dental implants: a histological and histomorphometrical study in the maxilla of pigs. Clin Oral Implants Res 2009 Nov;20(11):1247–53.
15. Gungor MB, et. al. An Overview of Zirconia Dental Implants: Basic Properties and Clinical Application of Three Cases. J Oral Implantol. 2014 Aug;40(4):485-94.
16. Thomé G, et. al. Clinical and radiographic success of injection-molded 2-piece zirconia implants submitted to immediate loading: A 12-month report of two cases. Clin Case Rep. 2021 Dec;9(12).
17. Thomé, Geninho et al. Osseointegration of a novel injection molded 2-piece ceramic dental implant: a study in minipigs. Clinical Oral Investigations, v. 25, 603-615, 2021.
18. Mellinghoff J. First clinical results of dental screw implants made of zirconium oxide [in German]. Z Zahnarztl Implantol. 2006; 22:288–293.
19. Kohal RJ, Knauf M, Larsson B, Sahlin H, Butz F. One-piece zirconia oral implants: one-year results from a prospective cohort study. 1. Single tooth replacement. J Clin Periodontol. 2012 Jun;39(6):590– 597.
20. Hashim D, Cionca N, Courvoisier DS, Mombelli A. A systematic review of the clinical survival of zirconia implants. Clin Oral Investig 2016 Sep;20(7):1403–1417.
21. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017 Feb;73(1):241-58.
22. Afrashtehfar KI, Del Fabbro M. Clinical performance of zirconia implants: A meta-review. J Prosthet Dent. 2020 Mar;123(3):419-26.
23. Borgonovo AE, Fabbri A, Vavassori V, Censi R, Maiorana C. Evaluation of the success criteria for zirconia dental implants: a four-year clinical and radiological study. Int J Dent 2013 Aug;463073.
24. Payer M, Arnetzl V, Kirmeier R, et al. Immediate provisional restoration of single-piece zirconia implants: a prospective case series – results after 24 months of clinical function. Clin Oral Implants Res. 2013 May;24(5):569-75.
