REGISTRO DOI: 10.5281/zenodo.10630237
Antonio C. A. MARANHÃO*; Guilherme V. CALEFFI*; Luan F. DE AZEVEDO*; Nicolas F. FONTANA*; Kayk B. CLIQUET*; Raphael A. DE SANTANA*; Vanda C. SANTOS*; Arthur D. C. SANTOS.**
ABSTRACT
Health care is shared and centralized in human behavior and wellbeing. Kefir-based products evidence possibility to cope inflammatory bowel diseases and promotion of a healthier lifestyle. Although, for both domestic and industrial productions, many information about kefir nutritional and health is known, aside the effects of specific substances on its growth is poorly known; this study aims, specifically, to investigate correlation of the interaction between sugar and colony growth. The chosen method allows systematic investigation and meaningful conclusions regarding quantitative aspects on non-target variables, such as temperature. four distinct sugar-to-kefir grain ratios undergoing the fermentation process with a of 12 hours between replacements, starting from the same initial point ( 0). As part of standardization, each vessel was uniformly covered with an approximately 0.2 mm thickness porous cloth, securely fastened using an elastic. Data were analyzed in order to assess variations related to sugar. By adding a non-volatile solute, the density of the mixture changes, converging toward the density of the more abundant compound. Accordingly, the density measured corroborates this change, indicating that a portion of the sugar remained dissolved after the 12 hours. The presence of dissolved sugar is noticeably through characteristics such as color, sweet taste and also viscosity.
INTRODUCTION
For centuries, people consciousness about healthy food has expanded, with more concerns on nutritional and probiotic values. Scientific evidence suggests a connection between disorder in intestinal microbiota and bowel inflammatory diseases[1,2], wherein consumption of so called “probiotics”, in certain amount, could promote its equilibrium by a dynamic interaction among intaken microrganisms in host and pathogenic bacteria[3-5].
Kefir, a probiotic fermented drink known for its several benefits[6] and rich nutritional values[7], is made by adding milk-kefir grains to cow, sheep or does milk. These grains exhibit a cauliflower-like appearance colored white to milky yellow. Composed of a matrix rich in polysaccharide and proteins, they host a diverse community of bacteria and yeasts, including Lactobacillus acidophilus, Lactobacillus kefiranofaciens, Lactobacillus casei, Lactobacillus fermentum, Lactobacillus kefirgranum, Lactobacillus kefir, Acetobacter aceti, Streptococcus cremoris, Saccharomyces cerevisiae, Saccharomyces lactis, Saccharomyces lipolytic, Candida kefir, and many others[8].
While much information about kefir nutritional and health is known, the effects of specific substances on its growth is poorly known, with this study aiming to specifically investigate the correlation of the interaction between sugar and colony growth. Such investigation appears as a useful material for fermentation industry by elucidating ways of production enhancing. Domestic production and science communication arises as a collateral aim, empowering people to produce at home kefir-based products with or without sugar.
MATERIAL AND METHODS
In addressing the central research question regarding the impact of sucrose on colony growth, the followed methodology aims to isolate variables during experiments. It is important to note that room temperature, a potential variable, could not be controlled. The recorded temperature measurements have a value of 23.89 +- 1.84°C. This approach allows systematic investigation and meaningful conclusions regarding quantitative aspects.
In the initial phase, four distinct sugar-to-kefir grain ratios (Sgmass/iKmass i sugar mass and initial kefir mass) were employed: 0:1, 1:1, 5:1, and 10:1, each replicated three times for subsequent statistical analysis. This experimental setup included a total of 12 glass vessels, with each vessel being labeled with its respective triplicate number (1, 2, and 3) and corresponding sugar-to-kefir grain ratio.
All vessels undergoing the fermentation process with a of 12 hours between replacements, starting from the same initial point (t0). As part of standardization, each vessel was uniformly covered with an approximately 0.2 mm thickness porous cloth, securely fastened using an elastic. Moreover, a consistent practice was upheld, maintaining a dark and covered environment for all the vessels throughout the fermentation process.
For all the experiments, the initial kefir mass (iKmass0) was set at 15 grams of grains, using 200 grams of UHT whole milk (Jussara, 3% fat) and white crystallized refined sugar (Caravelas), in proportion to initial kefir masses.
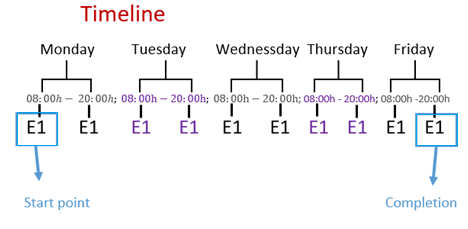
Figure 1: A timeline showing the milk and sugar replacements of all the experiments (E1) from the start point to the completion of the experiments.
Key replacements phases during the experiments were identified, occuring after the start point and following milk filtrations. At these points, milk was filtered with a plastic sieve with nylon threads and a stainless steel spoon, all sterilized with boiling water for 5-60 seconds. Measurements included room temperature, final kefir mass (fKmass), initial mass, as well as the pH, flavor, smell, density, and taste of an aliquot.
After data collection, statistical analysis using IBM SPSS Statistics (Version 29.0.2.0 (20)), employed the measurement of the p-value, the Pearson correlation and the mean yield in function of sugar mass.
RESULTS AND DISCUSSION
By adding a non-volatile solute, the density of the mixture changes, converging toward the density of the more abundant compound. Accordingly, the density measured corroborates this change, indicating that a portion of the sugar remained dissolved after the 12 hours. The presence of dissolved sugar is noticeably through characteristics such as color, sweet taste and also viscosity. The higher the sugar content, the more pronounced the yellowish color becomes.
Flavor analysis, although subjective, was assessed as the average of a values provided by three different individuals. The observed evaluations resulted in a sour taste ([5.3] on the sourness scale, with 10 being equivalent to the sourness of a lemon) for 0:1 proportion; a commercial yogurt-like flavors (slightly sour and sweet) for 1:1; a sweet taste for 5:1 ([5], on a scale where 10 equals to sweetness of condensed milk); and a sweet milk flavor for 10:1 ([8], also with 10 being equivalent to condensed milk).
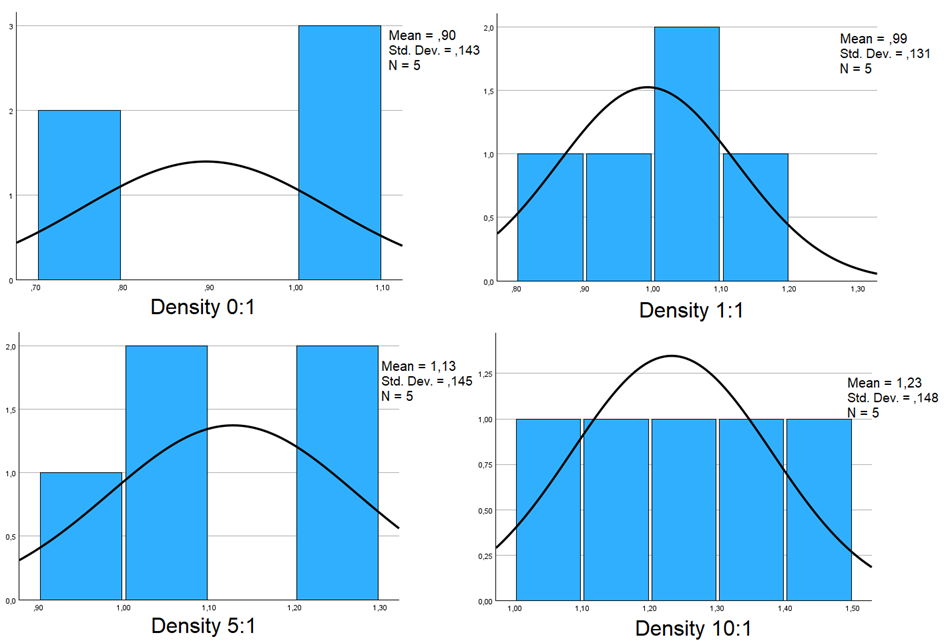
After the experiment’s data collection phase, mean numerical values were calculated for each of the three variables across the three triplicates. Specially, the final and initial kefir masses were used to derivate a new variable known as “yield” (Y). This yield represents the ratio of the kefir’s final mass at a point to the initial kefir mass of the experiment (fKmassn/iKmass0). The introduction of this yield not only offers a tangible measure of colony growth but also simplifies the interpretation of results and facilitates the calculation of Peason correlation.
As a rich source of glucose and fructose[9], commercial sugar was expected to enhance the energy supply for the colony. Accordingly, it was hypothesized (H1) that it would affect on colony growth, anticipating a positive correlation between sugar mass (Sgmass) and colony yield (Y). The statistical analysis, in support of H1, did reveal a correlation between yield and sugar mass. Unexpectedly, the Pearson correlation coefficient was negative, contradicting the initial expectations.
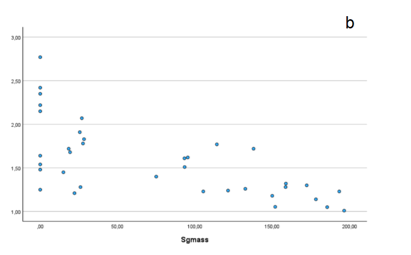
Figure 3: Pearson distribution visually showing the negative relation between sugar mass and yield.
CONCLUSION
Kefir, as a healthy beverage, is widely used for its probiotic effects, contributing to an improved quality of life for individuals seeking foods with additional benefits and disease prevention. Various kefir-selling companies have emerged around the world, including examples such as Wallaby Yogurt and Mapple Hill Creamery, occupying and expanding a niche of increasing health-conscious products. Therefore, methods for enhancing production, derived from data through the scientific method, as addressed in this study are deemed necessary. Although the limitation in temperature control and in a low universe number represents a loss of precision, the calculated negative Pearson correlation between sugar and yield reveals a curious non-intuitive dynamic interaction, paving the way for further research into the effects of specifics substances on kefir colonies.
REFERENCES
1. Shreiner, A. B., Kao, J. Y., & Young, V. B. (2015). The gut microbiome in health and in disease. Current opinion in gastroenterology, 31(1), 69.
2. Shi, N., Li, N., Duan, X., & Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system. Military Medical Research, 4, 1-7.
3. Ritchie, M. L., & Romanuk, T. N. (2012). A meta-analysis of probiotic efficacy for gastrointestinal diseases. PloS one, 7(4), e34938.
4. Collado, M. C., Isolauri, E., Salminen, S., & Sanz, Y. (2009). The impact of probiotic on gut health. Current drug metabolism, 10(1), 68-78.
5. Gill, H. S., & Guarner, F. (2004). Probiotics and human health: a clinical perspective. Postgraduate Medical Journal, 80(947), 516-526.
6. Carasi, P., Racedo, S. M., Jacquot, C., Romanin, D. E., Serradell, M. A., & Urdaci, M. C. (2015). Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. Journal of immunology research, 2015.
7. Ahmed, Z., Wang, Y., Ahmad, A., Khan, S. T., Nisa, M., Ahmad, H., & Afreen, A. (2013). Kefir and health: a contemporary perspective. Critical reviews in food science and nutrition, 53(5), 422-434.
8. Rosa, D. D., Dias, M. M., Grześkowiak, Ł. M., Reis, S. A., Conceição, L. L., & Maria do Carmo, G. P. (2017). Milk kefir: nutritional, microbiological and health benefits. Nutrition research reviews, 30(1), 82-96.
9. Vaccari, G., & Mantovani, G. (1995). Sucrose crystallisation. In Sucrose: properties and applications (pp. 33-74). Boston, MA: Springer US.
*Secretaria Estadual de Educação de São Paulo, Diretoria de Ensino de Santos, Escola Estadual Marquês de São Vicente
**Programa de Pós-Graduação em Ciências Farmacêuticas, Universidade Federal de São Paulo, Diadema – SP
