REGISTRO DOI: 10.69849/revistaft/ni10202410121122
Daniele Costa de Sousa5; Micael Porto Portela Lima1; Alexandre Almeida da Silva2; Natália Balbo Arantes Nogueira6; Isadora Vergamini Lamana6; Ana Carolina Gomes de Oliveira6; Larissa Tarraf Bertazzo6; Júlio César Claudino dos Santos1,2,3,4; Bruno de Paula Lima5
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by both motor and non-motor symptoms. Motor impairments, particularly in gait and balance, significantly reduce the quality of life in patients, increasing the risk of falls. Non-motor symptoms, including autonomic dysfunctions and peripheral neuropathy, are prevalent as the disease progresses. Peripheral neuropathy, present in 55% of PD patients, manifests with sensory loss, postural instability, and pain, further impairing motor function and increasing fall risk. This study aimed to adapt and test a simple method to identify small fiber neuropathy in PD patients, specifically using the Skin Wrinkling Test (SWT), and to correlate its findings with autonomic dysfunctions. Four PD patients were evaluated using questionnaires (Non-Motor Symptoms Questionnaire, COMPASS-31) and SWT. All patients showed alterations in the SWT, suggesting the presence of small fiber neuropathy, regardless of disease duration or comorbid conditions such as diabetes. Despite the small sample size, our findings support the SWT as a practical tool for assessing small fiber neuropathy in clinical practice. Further large-scale studies are needed to validate these results and their implications for diagnosing peripheral neuropathy in PD patients.
Keywords: Parkinson’s disease, peripheral neuropathy, small fiber neuropathy, autonomic dysfunction, Skin Wrinkling Test (SWT), motor symptoms, non-motor symptoms.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with motor and non-motor symptoms, affecting mainly older population (Rana et al., 2015). Motor impairment, with gait and balance deficits, is responsible mostly for the decrease in quality of life, as it progressively increases the risks of fall. Also, as disease progresses, multi-systemic disorders starts to appear, presenting itself in non-motor domains (orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor)(Rana et al., 2015). Peripheral neuropathy, once considered rare, is present in 55% of PD patients, compared to 8-9% in general population. As a result, it is one of the most prevalent non-motor symptoms in Parkinson’s disease patients, manifesting with postural instability, loss of peripheral sensation, weakness and pain (Corrà et al., 2023).
Peripheral neuropathy can be classified in small fiber neuropathy and large fiber neuropathy and its origin in PD patients can be linked to Levodopa intake, an intrinsic characteristic of PD or to patient concomitant chronic conditions, as diabetes, autoimmunity, or metabolic diseases (Kass-Iliyya et al., 2015).
Despite the intrinsic disfunction, peripheral neuropathy can lead to additional motor dysfunction, worsening global functional mobility and increasing the risks of falls, regardless of its etiology or the peripheral neuropathy type (Corrà et al., 2023). As a result, to improve gait in PD patients, recognition and directed treatment of peripheral neuropathy should be pursued. However, access to complementary exams to diagnose peripheral neuropathy are mostly difficult in underdeveloped countries, urging more accessible and practical diagnostic methods as skin biopsy (Leite Silva et al., 2023).
In order to facilitate the access to diagnosis and treatment, we specifically aimed to adapt and test a simple method to identify small fiber neuropathy in PD patients, to determine its prevalence and to compare with clinical features and non-motor symptoms of dysautonomia.
Methods
This study was approved by the Ethics Committee of Centro Universitário Christus, Unichristus CAAE: 58193322.2.0000.5049. It had an observational, longitudinal and qualitative design. It is epidemiological, population-based, conducted at the Physiotherapy School Clinic of the Christus University Center (Unichristus). The sample consisted of 04 individuals.
There were 4 interventional actions focusing on evaluating the quality of life of patients with Parkinson’s. Questionnaires exploring the research topic were applied (Non-Motor Symptoms Questionnaire, COMPASS 31, patient data) and a standardized questionnaire with the variables: age, sex, marital status, monthly income, education, time since diagnosis and presence of comorbidities , in addition to the Skin Wrinkling Test. The interviews were carried out by students and advisors. Those attending the Physiotherapy School Clinic who presented any changes in the evaluated parameters were advised to look for the family health unit closest to their residential area or a specialist.
Individuals aged 18 years or older, of both sexes, were included in the study. The exclusion criteria included people with some type of mental disorder that prevented them from understanding the procedures performed.
Dependent variables
COMPAS-31
The score aims to analyze the autonomic symptoms of individuals. The questionnaire consists of 31 questions and is divided into 6 domains: orthostatic, vasomotor, secretomotor, gastrointestinal, bladder and pupillomotor intolerance (TREISTER et al., 2015; SLETTEN et al., 2012). COMPASS-31 total scores had excellent internal validity (Cronbach’s α = 0.919), test-retest reliability (r(s) = 0.886; P < 0.001), and good convergent validity (r(s) = 0.474; P < 0.001 ). COMPASS-31 scores differed between subjects with or without small fiber polyneuropathy (Z = -3.296, P < 0.001) and demonstrated reasonable diagnostic accuracy.
Non-Motor Symptoms Questionnaire (NMSQ)
This is a 30-item questionnaire that indicates the presence or absence of non-motor symptoms in PD. NMSQ items are grouped into nine domains: gastrointestinal (8 items), urinary tract (2 items), sexual function (2 items), cardiovascular (2 items), apathy/attention/memory (3 items), hallucinations/delusions ( 2 items), depression/anxiety/anhedonia (2 items), sleep/fatigue (5 items), pain (1 item) and miscellaneous (3 items). For each item there is a yes or no answer. Parkinson’s patients completed the questionnaire translated, adapted and validated into Portuguese (https://pubmed.ncbi.nlm.nih.gov/32716283/).
Skin wrinkling test
This test was developed by Lewis and Pickering in 1936, being a simple and accessible way of evaluating the functions of fine autonomic and sensory fibers (TEOH; CHOW; WILDER-SMITH, 2008). The test consists of keeping one hand inside a reservoir containing 0.5 mmol/L of NaCl (sodium chloride) at a temperature of 40.5ºC, for 30 minutes. After the test, the evaluation is carried out by observing the wrinkling of four fingers, the thumb is not evaluated, thus a score is generated. The grading is easy to apply and skin wrinkling is directly correlated with abnormalities in biopsy studies, being considered the gold standard for evaluating the function of fine fibers (TEOH; CHOW; WILDER-SMITH, 2008). Skin wrinkling will be classified from 0 to 4, the grades were 0 being the absence of wrinkles; 1 wrinkle just noticeable on the fingertips; 2 two or fewer lines of superficial wrinkling; 3 three or more lines of deep wrinkling and 4 the wrinkling completely distorts the tip of the finger, with a score lower than 2, consistent with fine fiber dysfunction (TEOH; CHOW; WILDERSMITH, 2008; CLARK et al., 1984). Immersion of the hand will be carried out using a water reservoir and the temperature will be assessed using a thermometer. As the temperature drops, the water will be renewed. The skin wrinkle quantification test (TEC) has proven to be an alternative to more invasive exams and can be widely used for studies on the prevalence of small fiber neuropathies (GONDIM et al., 2014; TEOH; CHOW; WILDER-SMITH, 2008)
Independent variables
Sociodemographic: age, sex, marital status, monthly income, education, time since diagnosis and presence of comorbidities.
Results
Patients clinical profile
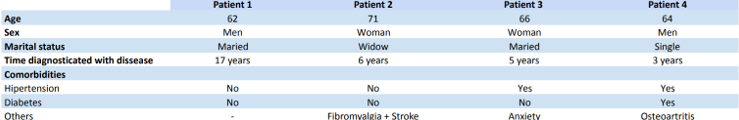
The comparison of sociodemographic characteristics and comorbidities of the 4 patients with Parkinson’s disease is described in table 1. Most patients also had some previous comorbidity.
Table 1: distribution of sociodemographic data and comorbidities among patients.
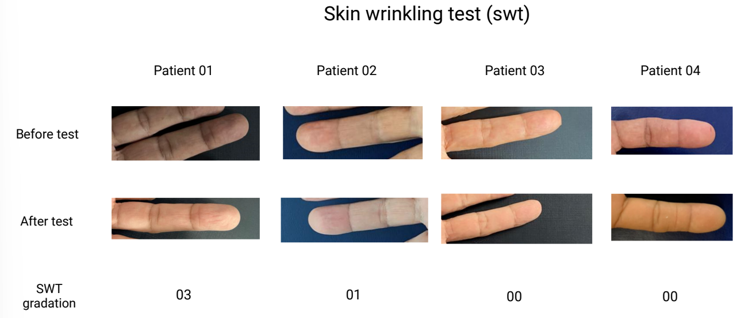
Skin wrinkling test avaliation
The patients’ hands were photographed before the SWT, and immediately after, they were immersed for a period of 30 minutes, during which the COMPASS-31 questionnaire (table 2) and NMSQ (table 3) were administered. After 30 minutes, they were photographed again, and skin wrinkling was analyzed according to the SWT.
Fig. 01. Skin wrinkling test
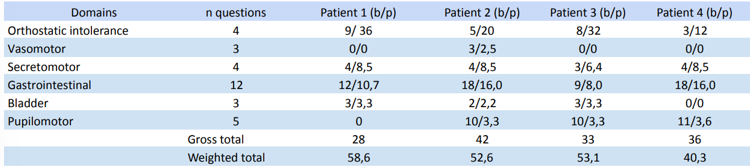
COMPAS-31 questionnaire
After data collection and tabulation, the weighted symptoms of the 4 patients were calculated.
Table 2: distribution of COMPAS-31 questionnaire results.
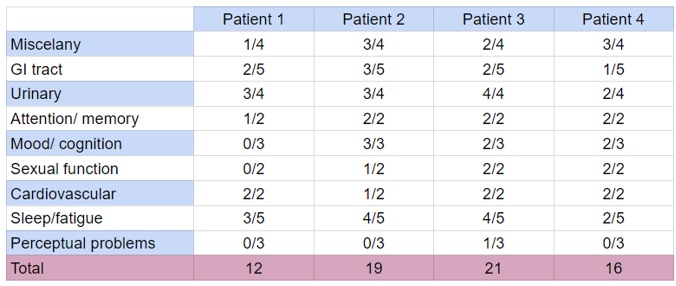
NMSQ questionnaire
Tabela 3: NMSQ questionnarie distribuition.
Discussion
This study describes four patients diagnosed with Parkinson disease accompanied on a physiotherapy’s clinic, during a clinical evaluation. As demonstrated in the literature, phosphorylated alpha-synuclein deposits have been found on autonomic nerves (colon, heart), and C fibers, a non myelinated fiber that is responsible for nociception, mechanical tact and the feeling of temperature and burning pain (Kass-Iliyya et al., 2015) Interestingly, the majority of patients (¾) answered question 10 (‘Unexplained pains, not due to known conditions such as arthritis’) as positive, demonstrating a correlation between the disease and afferent sensorial perception. Actually, its been shown that distal axons is more vulnerable to degeneration when compared to proximal sites, been demonstrated as an early symptom, preceding the accumulation in more proximal autonomic structures (Kass-Iliyya et al., 2015). In fact, patients 01 and 02, with longer diagnosis of PD (17 and 6 years), presented loss or change in the ability to taste or smell (question 02), correlating longer time of diagnosis and hyposmia, differently to what is found on studies with bigger demographic findings, which correlates hyposmia even before diagnosis and motor symptoms (Leite Silva et al., 2023). Actually, Braak defended the hypothesis that alpha-synuclein bodies are not only in the enteric nervous system, but also in the olfactory bulb, which ends up explaining the relationship between PD and olfactory dysfunction (De Rui et al., 2020).
Autonomic nonmotor symptoms are frequently associated with PD, as there is evidence in impaired cutaneous vasodilation capacity in PD patients. Sweating and thermoregulatory disturbances occur in almost two-thirds of patients, both hypo and hyperhidrosis. (Hirayama, 2006). Validating these findings, ¾ of our sample answered the question 28 (‘Excessive sweating’) as positive.
In previous studies from our group, it was extensively discussed about the early alpha-synuclein deposits on the enteric nervous system and glia (Claudino Dos Santos et al., 2023) preceding the motor clinical manifestations at least by 20 years. In fact, ¾ of our sample answered question 5 (Constipation (less than 3 bowel movements a week) or having to strain to pass a stool (faeces)) as positive. Actually, constipation is one of the first symptoms to be manifested on PD patients, followed by REM sleep behavior disorder and mood/ cognition disorders (Leite Silva et al., 2023). Bowel (fecal) incontinence (question 6), was found only on the patient with longer PD diagnosis.
Sleep disorders were present in our sample, accessed by the positive answers in questions 22-26, as well as mood/ cognition disorders, assessed by the positivity of questions 13, 16, 17. As described in literature, insomnia can be presented on 74–88% of patients with PD, being associated with difficulty maintaining sleep and sleep fragmentation (the most common sleep complaint in the population with PD), negatively impacting quality of life and being associated with further worsen of the progression of the disease (Leite Silva et al., 2023). Actually, as described on (Angelo Antonini & Pilleri, 2013) another sleep disorder presented on 15-50% of PD patients, may even preceding motor symptoms, is the REM sleep behavioral disorders (RBD), a parasomnia characterized by vivid dreams associated with harmful or disturbing behavior.
Urinary incontinence, which affects patients with PD twice as often as elderly patients without PD, occurring in 27–39% of all PD patients (Rana et al., 2015), as well as nocturia (caused by overactivity of the urinary bladder detrusor muscle), was accessed by questions 7, 8, 9 and 27, demonstrating positivity in our sample. Furthermore, contributing for the worsening of PD quality of life, sexual dysfunctions play an important role, even contributing to the onset of depressive symptoms (Weintraub et al., 2022). In this study, sexual dysfunction was measured by questions 18 and 19, also demonstrating positivity.
An important confounding factor in our study, due to the small size, is due to patient 01. This patient, although diagnosed at an early age (45 years), having 17 years of disease, had an increased overall health (no other diagnosed diseases), less symptoms (table 2, raw score of 28) and better punctuation on the Skin wrinkling test (03). This could, in part, be explained due to the fact that this patient had a previous story of physical activity (beach tennis and table tennis)and controlled medication. Interestingly, a recent Cohort study published on Neurology, found that average regular overall physical activity levels over time were significantly associated with slower symptoms deterioration in patients with early parkinson disease (Tsukita et al., 2022), an association that may attenuate the symptoms, although patient 01 presented worse autonomic symptoms considering COMPASS-31 weighted score (58,6). Another confounding factor implies on patient 04, that although he has the smallest diagnosis time (03 years), he already has the most severe SWT score. As a result, we attribute the rapid prevalence of his neuropathy due to his co-occurring diabetes, contributing even before the diagnosis to the worsening of symptoms (Feldman et al., 2019).
As a limitation, although we didn’t complete a full autonomic test and made skin biopsies, the advantages of our study consist of the additional clinical evaluations, using COMPASS-31 and NMSQ, in addition to the SWT, to evaluate our patients, differently to what was made on other studies using only SWT (de Araújo et al., 2016). Although it is difficult to extrapolate findings to the general population due to our small sample, 100% of our sample demonstrated alterations on the SWT, validating its use in clinical practice for evaluation of PD small fiber neuropathy.
Conclusion
In summary, small fiber peripheral neuropathy and association with autonomic dysfunctions is presented in PD patients independently of the time of their diagnosis. As a result, SWT was positively associated with autonomic dysfunctions, although in our sample neuropathy itself could also have other origins, as diabetic neuropathy. However, large sample studies are needed to broad our results to a health worker clinical practice.
References
Angelo Antonini, K. K., Daniela Calandrella, Marcelo Merello, & Pilleri, M. (2013). Effects of rotigotine on Parkinson’s disease-related sleep disturbances. Expert Opinion on Pharmacotherapy, 14(18), 2571–2580. https://doi.org/10.1517/14656566.2013.849692
Claudino Dos Santos, J. C., Lima, M. P. P., Brito, G. A. D. C., & Viana, G. S. D. B. (2023). Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Research Reviews, 84, 101812. https://doi.org/10.1016/j.arr.2022.101812
Corrà, M. F., Vila-Chã, N., Sardoeira, A., Hansen, C., Sousa, A. P., Reis, I., Sambayeta, F., Damásio, J., Calejo, M., Schicketmueller, A., Laranjinha, I., Salgado, P., Taipa, R., Magalhães, R., Correia, M., Maetzler, W., & Maia, L. F. (2023). Peripheral neuropathy in Parkinson’s disease: Prevalence and functional impact on gait and balance. Brain, 146(1), 225–236. https://doi.org/10.1093/brain/awac026
de Araújo, D. F., de Melo Neto, A. P., Oliveira, Í. S. C., Brito, B. S., de Araújo, I. T., Barros, I. S., Lima, J. W. O., Horta, W. G., & Gondim, F. de A. A. (2016). Small (autonomic) and large fiber neuropathy in Parkinson disease and parkinsonism. BMC Neurology, 16, 139. https://doi.org/10.1186/s12883-016-0667-3
De Rui, M., Inelmen, E. M., Trevisan, C., Pigozzo, S., Manzato, E., & Sergi, G. (2020). Parkinson’s disease and the non-motor symptoms: Hyposmia, weight loss, osteosarcopenia. Aging Clinical and Experimental Research, 32(7), 1211–1218. https://doi.org/10.1007/s40520-020-01470-x
Feldman, E. L., Callaghan, B. C., Pop-Busui, R., Zochodne, D. W., Wright, D. E., Bennett, D. L., Bril, V., Russell, J. W., & Viswanathan, V. (2019). Diabetic neuropathy. Nature Reviews Disease Primers, 5(1), 41. https://doi.org/10.1038/s41572-019-0092-1
Hirayama, M. (2006). Sweating dysfunctions in Parkinson’s disease. Journal of Neurology, 253(7), vii42–vii47. https://doi.org/10.1007/s00415-006-7010-7
Kass-Iliyya, L., Javed, S., Gosal, D., Kobylecki, C., Marshall, A., Petropoulos, I. N., Ponirakis, G., Tavakoli, M., Ferdousi, M., Chaudhuri, K. R., Jeziorska, M., Malik, R. A., & Silverdale, M. A. (2015). Small fiber neuropathy in Parkinson’s disease: A clinical, pathological and corneal confocal microscopy study. Parkinsonism & Related Disorders, 21(12), 1454–1460. https://doi.org/10.1016/j.parkreldis.2015.10.019
Leite Silva, A. B. R., Gonçalves De Oliveira, R. W., Diógenes, G. P., De Castro Aguiar, M. F., Sallem, C. C., Lima, M. P. P., De Albuquerque Filho, L. B., Peixoto De Medeiros, S. D., Penido De Mendonça, L. L., De Santiago Filho, P. C., Nones, D. P., Da Silva Cardoso, P. M. M., Ribas, M. Z., Galvão, S. L., Gomes, G. F., Bezerra De Menezes, A. R., Dos Santos, N. L., Mororó, V. M., Duarte, F. S., & Dos Santos, J. C. C. (2023). Premotor, nonmotor and motor symptoms of Parkinson’s Disease: A new clinical state of the art. Ageing Research Reviews, 84, 101834. https://doi.org/10.1016/j.arr.2022.101834
Rana, A. Q., Ahmed, U. S., Chaudry, Z. M., & Vasan, S. (2015). Parkinson’s disease: A review of non-motor symptoms. Expert Review of Neurotherapeutics, 15(5), 549–562. https://doi.org/10.1586/14737175.2015.1038244
Tsukita, K., Sakamaki-Tsukita, H., & Takahashi, R. (2022). Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With Early Parkinson Disease. Neurology, 98(8), e859–e871. https://doi.org/10.1212/WNL.0000000000013218
Weintraub, D., Aarsland, D., Chaudhuri, K. R., Dobkin, R. D., Leentjens, A. F., Rodriguez-Violante, M., & Schrag, A. (2022). The neuropsychiatry of Parkinson’s disease: Advances and challenges. The Lancet. Neurology, 21(1), 89–102. https://doi.org/10.1016/S1474-4422(21)00330-6
1Medical School of the Christus University Center – UNICHRISTUS, Fortaleza, CE, Brazil.
2School of Physiotherapy of the Christus University Center – UNICHRISTUS, Fortaleza, CE, Brazil.
3Graduate Program in Morphofunctional Sciences, Federal University of Ceará – UFC, Fortaleza, CE, Brazil.
4Morphology Department of the Federal University of Ceará – UFC, Fortaleza, CE, Brazil.
5Medical School of the Federal University of Amapá, UNIFAP, Macapá, AP, Brazil.
6Medical School of the Barão of Mauá University Center – CBM, Ribeirão Preto, SP, Brazil.
