REGISTRO DOI: 10.69849/revistaft/ra10202411061804
Gisele Marlene Maciag1
André Pessoa Silva de Bastos2
Antônio Jakeulmo Nunes3
Nirvana Ramos de Araújo4
Isabela Maria Rios Malta5
Flávia Gorete Monte de Morais6
Fabrício da Silva Freitas7
Levi Amorim de Carvalho Rodrigues8
Gabriel dos Santos Silva9
Ayana Rocha Pôrto Mousinho10
Summary
Introduction: Obesity is a public health condition that results in adverse health impacts and, together with overweight, is one of the main risk factors for the development of type 2 diabetes mellitus. Semaglutide , an analogue of glucagon-like peptide 1 (GLP-1) , has emerged as a promising therapeutic option in the management of obesity, since it can be prescribed for both glycemic control and weight reduction.Methodology: An integrative review was carried out in the databases indexed by the Virtual Health Library (VHL), using as inclusion criteria primary articles published in 2024 in English, with 8 works selected. Results and discussion: Semaglutide has been shown to be an effective pharmacological treatment for patients with type 2 Diabetes Mellitus. Both its weekly subcutaneous and daily oral presentation were able to reduce the average weight and BMI of patients with a variation between 3% and 6% in 3 to 12 months, with the reduction being proportional to the dosage. However, adverse effects were frequent in patients, mostly mild and involving nausea, diarrhea, and constipation, sometimes sufficient to impair adherence. Conclusion : Semaglutide is a safe and effective pharmacological option for the treatment of obesity and overweight in type 2 diabetics.
Keywords: Obesity Control. Incretions; Peptide Receptor Agonists. Diabetes Mellitus.
INTRODUCTION
Obesity is a chronic condition characterized by excessive accumulation of body fat, which results in adverse health impacts and increases the risk of several diseases, including type 2 diabetes (T2D), hypertension, cardiovascular disease, and certain types of cancer ( Lingvay et al, 2024). It is considered one of the main risk factors for the development of T2D, a metabolic disease characterized by insulin resistance and/or impaired secretion of this hormone, which leads to high blood glucose levels (Wysocki et al, 2024). The relationship between obesity and T2D is bidirectional: the presence of obesity aggravates insulin resistance, while inadequate glucose control in diabetic patients can contribute to weight gain, creating a vicious cycle that is difficult to break. (Ahmad et al, 2024).
Obesity is a global public health problem, with increasing prevalence worldwide. According to the World Health Organization (WHO), more than 650 million adults were obese in 2016, and projections indicate that these numbers will continue to rise (Spanholi et al, 2024 ; Mahfouz, 2024). The condition is associated with reduced quality of life, increased healthcare costs, and higher mortality. Implementing effective therapies is essential to control this epidemic and reduce associated complications (Shafiee et al, 2024).
Non-pharmacological treatments, such as dietary changes, increased physical activity and behavioural interventions, are fundamental in the initial management of obesity. However, in many cases, these methods are not sufficient to achieve and maintain clinically meaningful weight loss, especially in patients with refractory obesity (Assyov et al, 2024). This highlights the need for pharmacological interventions, which play an important role in the treatment of individuals who do not respond adequately to lifestyle changes (Tchang et al, 2024).
Semaglutide , a glucagon-like peptide 1 (GLP-1) analogue, has emerged as a promising therapeutic option in the management of obesity, especially in patients with T2DM ( Irfan , 2024). Belonging to the class of GLP-1 receptor agonists, semaglutide mimics the effects of this hormone by promoting insulin secretion in response to rising blood glucose, delaying gastric emptying and reducing appetite (Seetharaman et al, 2024). These mechanisms contribute to both glycaemic control and weight reduction, which suggest semaglutide as an innovative and effective approach for the treatment of individuals with obesity associated with T2DM ( Carri,2024).
However, like any drug therapy, semaglutide is associated with adverse effects, the most common of which are nausea, vomiting, diarrhea, and constipation. These effects, although generally transient, can limit treatment adherence, highlighting the need for appropriate monitoring and management during drug use (Sillassen et al, 2024). The use of semaglutide thus represents a significant advance in the treatment of obesity and T2DM ( Alkhalifah et al, 2024).
In this context, the present study aims to evaluate the efficacy of semaglutide as a pharmacological therapy for obesity in individuals with T2DM, as well as to define the adverse effects profile of this drug.
METHODOLOGY
A bibliographic review aims to carry out and analyze articles and studies on a given subject, being an important piece for the development of future studies and research. (Gonçalves, 2019). Thus, this study is an integrative review, carried out through searches in the databases indexed to the brasilian Virtual Health Library (VHL), which together allow access to the Scientific platforms Electronic Library Online (SciELO) , Medical Literature Analysis and Retrieval System Online (Medline) and Latin American and Caribbean Literature in Health Sciences (LILACS). The inclusion criteria were original articles available in full, published in the year 2024, in English, as exclusion criteria, duplicate articles, unrelated to the topic and literature reviews. The searches were carried out using the following terms in English as descriptors: “ Obesity Management”, “GLP 1 Analogs ”, “ Incretins ”, “ Diabetes Mellitus” being the first combined with the other two, using the Boolean operator “ And ”.
Initially, a screening was performed by analyzing the titles of the articles collected in the database, and 18 articles were selected. Of this total, an analysis of the abstracts of their abstracts was performed for final selection. Thus, 10 articles were excluded, leaving 8 for content evaluation, which comprised this research. The organizational chart below summarizes the selection of articles for the present study.
Figure 1 – Article selection chart.
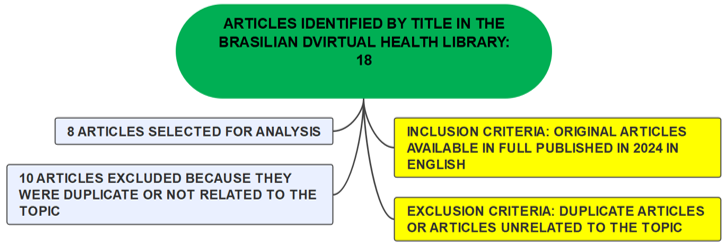
Source: The authors
RESULTS
Only 8 articles met the inclusion criteria. For better understanding, the studies were organized by description of some characteristics, such as: first author, year of publication, journal, type of study and objectives as can be seen in Table 1 below.
Table 1 – Distribution of research data in relation to author, year of publication, journal and purpose of publication.
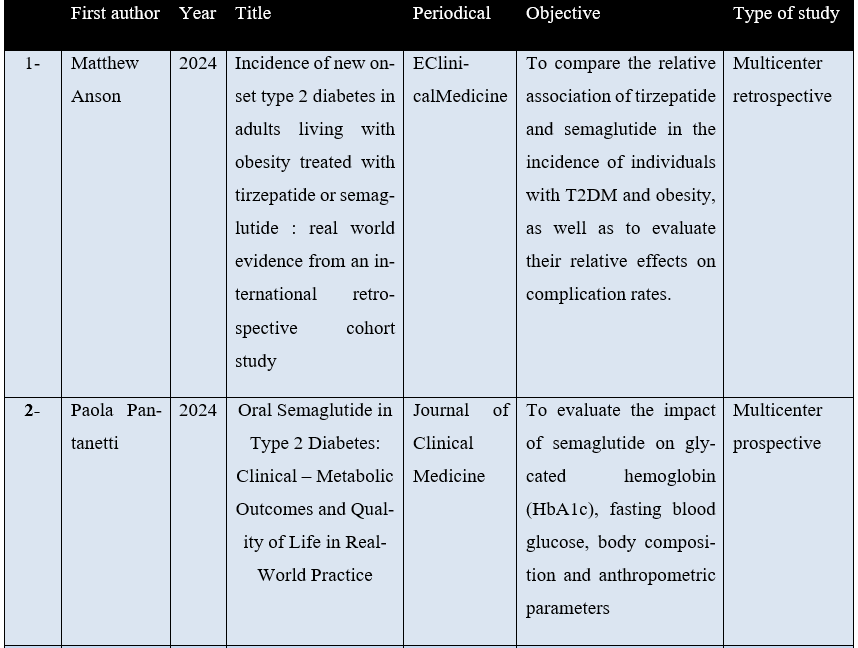
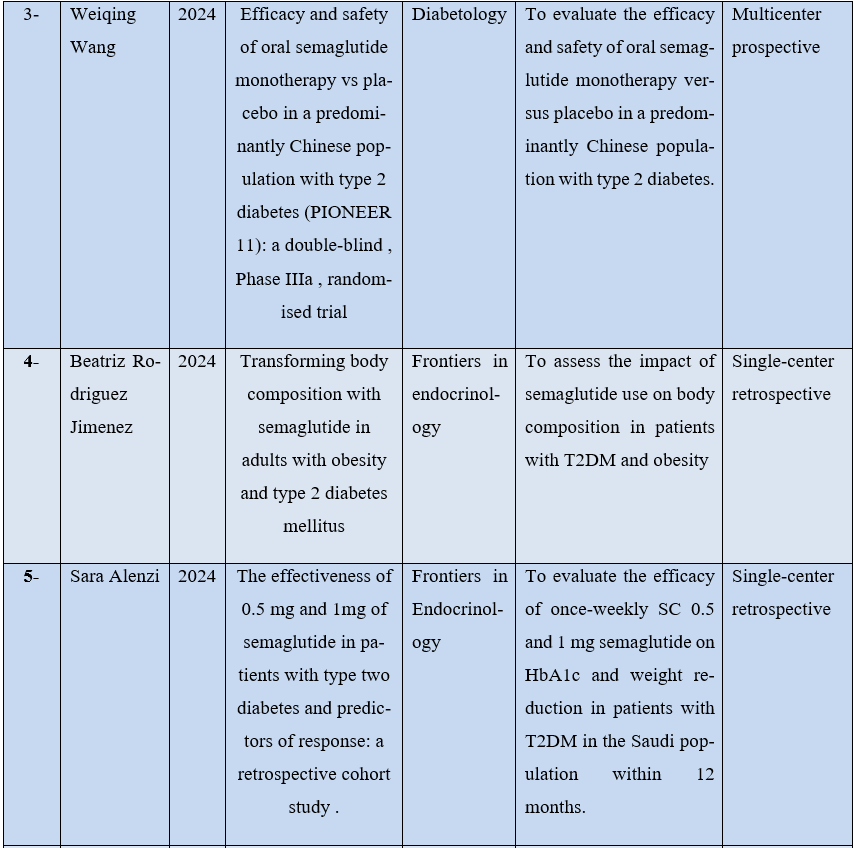
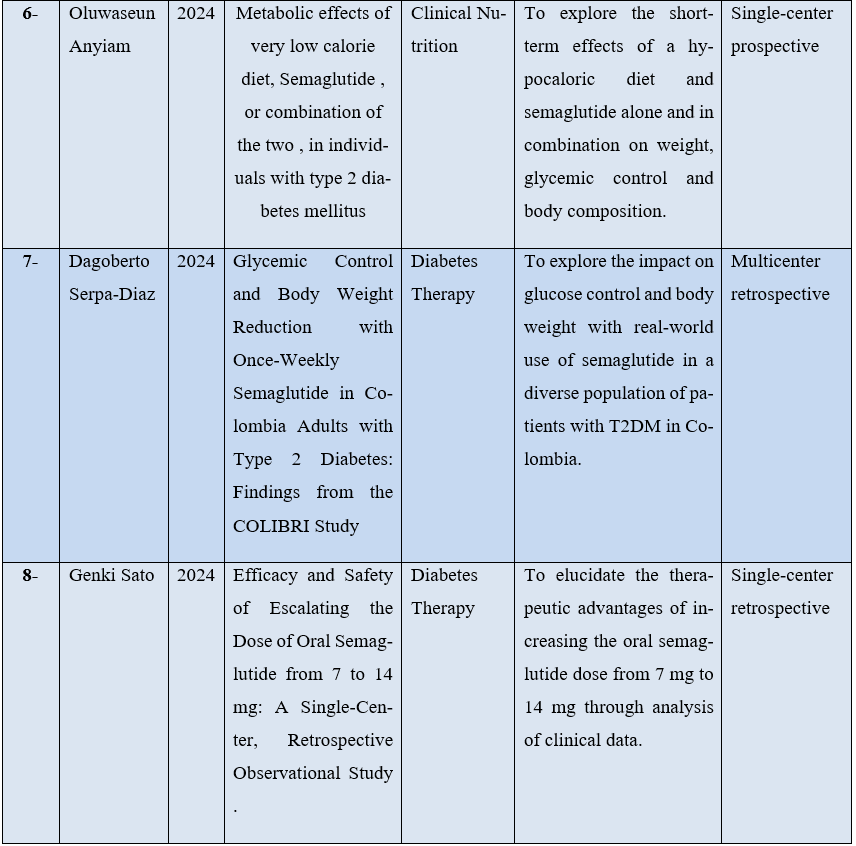
Source: The authors.
DATA ANALYSIS
Effectiveness of oral presentation
In the Italian multicenter prospective study by Pantanetti et al. (2024), 61 patients with T2DM with a mean age of 61 years were evaluated 6 months after using oral semaglutide 14 mg/day for HbA1c, body composition, anthropometric parameters, and the Diabetes Treatment Satisfaction Questionnaire (DTSQ). Initially, the mean body weight was 89.19 kg (± 5.84), after therapy it was 86.1 kg, showing a difference of -2.2 kg on average. Also in this context, the initial mean body mass index (BMI) was 30.81 (± 1.96), with 56.67% of patients being obese (BMI ≥ 30), and after therapy, it was 29.61, that is, an average reduction of -1.19. Therefore, even in a short period, oral semaglutide was shown to be effective in weight loss in obese or overweight individuals. Similar results were described by Wang et al. (2024) when they published a prospective, double-blind, multicenter study carried out in 5 countries, where 489 patients with T2DM were randomized to receive oral semaglutide 1x daily at a dose of 3 mg (group 1, n = 130), 7 mg (group 2, n = 130), 14 mg (group 3, n = 130) or placebo (group 4, n = 131) for 26 weeks, in order to assess HbA1c and body weight, with the groups having a mean age of 52 years. At baseline, mean body weight was 79.6 kg, significantly greater reductions in body weight were observed for oral semaglutide 7 mg (-1.2 kg) and 14 mg (-2.0 kg) than for placebo (-0.8 kg) at week 26 but not for oral semaglutide 3 mg (-0.0 kg).
Using a similar methodology, in Japan, 57 adults with a mean age of 56.1 years, mean BMI of 32 kg/m2 (± 7.2) with T2DM received oral semaglutide 14 mg for 6 months to assess their clinical outcomes also in HbA1c and metabolic parameters. The mean initial weight was a significant reduction in body weight of 90.0 kg (± 20.5) and was reduced to 88.2 kg (± 21.4), which resulted in a mean reduction of − 2.0 kg (± 4.4). In this context, 41.2% (n = 21) reduced their weight by more than 3% and 15% (n = 8) reduced by more than 5%, attesting to the effectiveness of the medication in weight loss even in those who are not obese (Sato; Uchino ; Hirose , 2024). These findings were consistent with those observed in the North American multicenter retrospective cohort study by Anson et al. (2024), where several patients with or without T2DM were followed for 12 months to evaluate the effect of semaglutide and tirzepatide (a drug with similar indications and pharmacodynamics to the first) on weight loss and HBA1c control, although they had already been using these for up to 6 months. The 4223 patients with T2DM using semaglutide initially had a mean age of 54 years and a mean weight of 105.1 kg (± 25.9); after 12 months of therapy, they had a weight loss of -4.8 kg.
The findings of the aforementioned studies reinforce the efficacy of oral semaglutide in reducing weight and improving glycemic control in patients with type 2 diabetes (T2DM). The study by Pantanetti et al. (2024) showed that, even in a period of only six months, the significant reduction in weight and BMI indicates a clear benefit in the body composition of patients. These results are corroborated by Wang et al. (2024), who observed more pronounced weight reductions with higher doses of semaglutide , suggesting a dose-response effect. Consistently, the findings in Japan and in the North American cohort point to the efficacy of semaglutide , even in different populations, with significant weight reductions, especially with long-term use. This highlights the promising role of oral semaglutide in T2DM therapy, not only in glycemic control, but also in the management of overweight and obesity.
Efficacy of subcutaneous presentation
In Colombia, Serpadiaz et al. (2024) published a multicenter retrospective study to assess the outcomes in body weight change and HbA1c from baseline after the use of weekly subcutaneous semaglutide for 6 months. In this sense, the study population consisted of 225 patients with a mean age of 57 (± 11.7) years, with DM2 and overweight or obesity, with a median body weight of 86 kg; After the intervention, the median weight of the group reduced to 82 kg, which represents a median weight loss of -4.04 kg. These findings are consistent with the results of a prospective English study in which 10 individuals who were also obese and had DM2 received weekly subcutaneous semaglutide escalated to 1 mg for 12 weeks. In this context, initially the average age of the participants was 61.1 years (± 3.0) and the average weight was 103.2 kg (± 4.1). After the intervention, this reduced to 97.1 kg (± 4), demonstrating an average loss of 6 kg in the study group or 6% of the mass. In the same sense, the average BMI also showed a reduction, being reduced from 35.0 (± 1.6) to 33.0 (± 1.6) ( Anyiam et al, 2024).
In the Saudi population, a retrospective cohort study was conducted with 363 patients with a mean age of 52.6 (± 8.5) with T2DM and obesity to evaluate the efficacy of using semaglutide via SC 0.5 mg (n = 105) and 1 mg weekly (n = 258) on HbA1c and weight reduction in 12 months, whose mean total weight was 94 kg (± 15.7). In this context, the lowest dose showed a mean reduction of -6.1 kg (± 5), while the highest dose showed a reduction of -6.2 kg (± 4.4), with similar efficacy, suggesting that the lowest dose should be indicated and consider increasing according to the patient’s response ( Alenzi et al, 2024).
The findings of the studies conducted by Serpa-Diaz et al. (2024), Anyiam et al. (2024), and Alenzi et al. (2024) indicate that subcutaneous semaglutide is effective in reducing body weight and BMI in patients with T2DM and overweight or obesity. The results demonstrated significant weight loss across different populations and intervention periods, with median and mean reductions ranging from 4 kg to 6.2 kg. These data are consistent regardless of geographic context, suggesting that semaglutide has a robust therapeutic profile for weight management in diabetic individuals. Notably, the Saudi study highlighted that lower doses (0.5 mg) may be as effective as higher doses (1 mg) in reducing weight, which may optimize treatment while minimizing the risk of adverse effects associated with higher doses. Similarly, the reductions in BMI observed in several studies reinforce the clinical utility of subcutaneous semaglutide in the treatment of obesity associated with T2DM. However , there remains a need for further investigation to establish the minimum effective dose and assess the maintenance of long-term benefits. In this regard, these findings support the use of semaglutide as a promising therapeutic option for weight control in diverse populations.
Comparison of subcutaneous presentation with oral presentation
Jimenzes et al. (2024) in their 6-month retrospective study with 88 adults with T2DM and obesity evaluated the effect of daily oral semaglutide escalated to 1mg (Group 1 n=33) and weekly subcutaneous escalated to 14mg (Group 2, n=55) on body composition, through the analysis of electrical bioimpedance. The initial BMI of group 2 was 40.1 kg/m2 , at the end of the period, it showed a reduction to 36.1 kg/m2 , representing a decrease of -3.7 kg/m2. In the same context, the initial average weight was 109.2kg and after medication, 98.5kg, representing a decrease of -10kg on average. The initial BMI of group 1 was 34.2, showed a reduction to 31, representing a decrease of -3.1 points. In this same context, the initial average weight was 94.8 kg and after medication, 86.2 kg, representing a decrease of -8.6 kg on average. This study excluded patients who felt adverse effects, which made it difficult to indicate one presentation in relation to another based on this factor. These results indicate that both oral and subcutaneous semaglutide are effective in reducing weight and BMI in adults with T2DM and obesity, with similar reductions in both groups after six months of treatment. Although there are few direct comparisons in the literature between the two forms of administration, this study contributes important evidence showing that clinical efficacy in weight loss is comparable, regardless of the route of administration. It is suggested that the main advantage of subcutaneous semaglutide lies in its weekly dosing, which may facilitate adherence to treatment, especially in patients who prefer a lower dosing frequency. However, it is important to consider the limitation of the study, which excluded patients who reported adverse effects, which may influence the generalizability of the results.
Adverse Effects
Regarding the adverse effects of the use of oral semaglutide , in the work published with 489 patients with type 2 diabetes in Asian countries, being proportional to the daily dose, gastrointestinal disorders were more frequently reported in therapy (3 mg: 16.2%, 7 mg: 32.3% and 14 mg: 31.8%) than with placebo (9.2%). Of these, diarrhea and nausea were the most common, although most cases were mild and of short duration, presenting respectively according to the dose 3mg: 3.1%, 7mg: 9.2% and 14mg: 9.3% and 3mg: 4.6%, 7mg: 3.1% and 14mg: 6.2% (Wang et al, 2024). In the Japanese study with 57 adults by Sato; Uchino and Hirose (2024), 27.3% (n=18) reported some adverse effect, of this total, 7.6% (n=5) reported nausea and 1.5% (n=1) reported diarrhea.
Regarding weekly subcutaneous semaglutide , in the English study by Anyiam et al. (2024) with 10 type 2 diabetic patients at a dose of 1 mg, 45% (n=5) presented mild nausea, 36% reported constipation, 27.3% presented severe abdominal pain and 27.3% abdominal distension.
The findings suggest that gastrointestinal adverse effects are common with semaglutide , both oral and subcutaneous, with frequency and severity generally related to dose, but generally mild and transient. In the Asian study, gastrointestinal disturbances were more prevalent with higher doses of oral semaglutide , while in the Japanese study, a significant percentage of patients reported adverse effects. Similarly, for subcutaneous semaglutide , adverse effects were also common, with nausea and abdominal discomfort standing out. However, the small sample size of the studies, especially in the case of the English study, limits the generalizability of the results. There is a lack of larger studies that evaluate the true epidemiology of adverse effects, both for the oral and subcutaneous forms, in order to determine the safety profile more accurately and guide clinical practice.
CONCLUSION
Semaglutide , both in oral and subcutaneous forms, is an effective intervention for weight management and glycemic control in patients with type 2 diabetes and obesity. Efficacy was superior to placebo for the 7 mg and 14 mg oral doses, with the 14 mg dose showing slightly better results. In the subcutaneous form, the 0.5 mg and 1 mg doses showed similar efficacy, demonstrating that lower doses may be feasible for many patients. In this regard, adverse effects, predominantly gastrointestinal such as nausea and constipation, were generally mild and self-limiting. It is noteworthy that the response to treatment was consistent across different geographic locations, reinforcing its applicability in diverse populations. Despite the positive evidence, most studies involved individuals between 40 and 60 years of age, indicating the need for further investigations in other age groups to better understand the safety and efficacy profile.
REFERENCES
ALENZI, Sara et al. The effectiveness of 0.5 mg and 1mg of semaglutide in patients with type two diabetes and predictors of response: a retrospective cohort study . Frontiers in Endocrinology , vol. 15, p. 1395651, 2024.
ANYIAM, Oluwaseun et al. Metabolic effects of very low calorie diet, Se- maglutide , or combination of the two , in individuals with type 2 diabetes mellitus. Clinical Nutrition , vol. 43, no. 8, p. 1907-1913, 2024.
AHMAD, Muhammad Saeed et al. Exploring the Interactions between Obesity and Diabetes: Implications for Understanding Metabolic Dysregulation in a Saudi Arabian Adult Population . Journal of Proteome Research , vol. 23, no. 2, p. 809-821, 2024.
ANSON, Matthew et al. Incidence of new onset type 2 diabetes in adults living with obesity treated with tirzepatide or semaglutide : real world evidence from an international retrospective cohort study . EClinicalMedicine , v. 75, 2024.
ALKHALIFAH, Mohammed et al. Semaglutide for the management of diabesity : The real-world experience . World Journal of Methodology , vol. 14, no. 3, p. 91832, 2024. ASSYOV, Yavor et al. Nutritional Management and Physical Activity in the Treatment of Sarcopenic Obesity : A Review of the Literature . Nutrients , vol. 16, no. 15, p. 2560, 2024.
CARRIS, Nicholas W. et al. Discontinuing semaglutide after weight loss : Strategy for weight maintenance and a possible new side effect . Canadian Journal of Physiology and Pharmacology , vol. 102, no. 6, p. 391-395, 2024.
IRFAN, Hamza . Obesity , cardiovascular disease , and the promising role of semaglutide : Insights from the SELECT trial . Current Problems in Cardiology , vol. 49, n. 1, p. 102060, 2024.
LINGVAY, Ildiko et al. Obesity in adults . The Lancet, vol. 404, no. 10456, p. 972-987, 2024.
MAHFOUZ, Ahmed A. et al. Prevalence of Obesity and Associated Dietary Habits among Medical Students at King Khalid University , Southwestern Saudi Arabia . Medicine, vol. 60, no. 3, p. 347, 2024.
RODRÍGUEZ JIMÉNEZ, Beatriz et al. Transforming body composition with semaglutide in adults with obesity and type 2 diabetes mellitus. Frontiers in Endocrinology , vol. 15, p. 1386542, 2024.
SEETHARAMAN, Rajmohan et al. IcoSema : Unveiling the future of diabetes management from a clinical pharmacology perspective. Journal of Basic and Clinical Physiology and Pharmacology , n. 0, 2024.
SHAFIEE, Arman et al. Global prevalence of obesity and overweight among medical students : A systematic review and meta- analysis . BMC Public Health, vol. 24, no. 1, p. 1673, 2024. SILLASSEN, Christina Dam Bjerregaard et al. Adverse effects with semaglutide : A protocol for a systematic review with meta- analysis and trial sequential analysis . BMJ Open, vol. 14, no. 6, p. e084190, 2024.
SERPA-DÍAZ, Dagoberto et al. Glycemic Control and Body Weight Reduction with Once-Weekly Semaglutide in Colombia Adults with Type 2 Diabetes: Findings from the COLIBRI Study . Diabetes Therapy , vol. 15, no. 6, p. 1451-1460, 2024.
SPANHOLI, Mariana Winck et al. Trends in the prevalence of obesity , overweight , and thinness among schoolchildren aged 7–14 years from southern Brazil (2002–2019). American Journal of Human Biology , vol. 36, no. 4, p. e24013, 2024.
PANTANETTI, Paola et al. Oral Semaglutide in Type 2 Diabetes: Clinical – Metabolic Outcomes and Quality of Life in Real-World Practice . Journal of Clinical Medicine, vol. 13, no. 16, p. 4752, 2024.
TCHANG, Beverly G. et al. Pharmacologic treatment of overweight and obesity in adults . Endotext [Internet], 2024.
WANG, Weiqing et al. Efficacy and safety of oral semaglutide monotherapy vs placebo in a predominantly Chinese population with type 2 diabetes (PIONEER 11): a double-blind , Phase IIIa , randomised trial . Diabetologia , v. 67, no. 9, p. 1783-1799, 2024.
WYSOCKI, Michał et al. Analysis of Changes in Glucose and Lipid Metabolism in Patients with Clinically Severe Obesity and Type 2 Undergoing Diabetes Mellitus Laparoscopic Sleeve Gastrectomy — Prospective Observational Study . Obesity Surgery , vol. 34, no. 2, p. 467-478, 2024.
1Graduated in medicine from the Universidade Comunitária da Região de Chapecó – Unochapecó
2,3,6,8,9Student of the Higher Education Course in Medicine at the Universidade Federal do Delta do Parnaíba – UFDPar
4Graduated in medicine from the Instituto de Educação Superior do Vale do Parnaíba
7Student of the Higher Education Course in Medicine at the Universidade Federal do Ceará – UFC
10Student of the Higher Education Course in Medicine at Instituto de Educação Superior do Vale do Parnaíba – IESVAP
