PREVALENCE OF HEMOPARASITES EHRLICHIA CANIS, ANAPLASMA PLATYS, AND BABESIA VOGELI IN DOMICILED DOGS IN THE METROPOLITAN REGION OF BELÉM/PA: A RETROSPECTIVE STUDY
REGISTRO DOI: 10.69849/revistaft/ma10202509141005
Phâmella Vasco Magalhães1
Monique Araújo Luz2
Rodrigo Rodrigues Virgolino3
Leopoldo Augusto Moraes4
Evonnildo Costa Gonçalves5
Délia Cristina Figueira Aguiar5
Resumo
Abstract: Canine monocytic ehrlichiosis, canine thrombocytic anaplasmosis, and canine babesiosis are diseases caused, respectively, by bioagents Ehrlichia canis, Anaplasma platys e Babesia vogeli, that have gained great relevance due to its zoonotic character. The hot and humid climate in the Amazon region favors the proliferation of hematophagous vectors. The present study investigated the prevalence of E. canis , A. platys, and B. vogeli during the years 2013 to 2017 through the analysis of the database with results of tests carried out on blood samples of 6,593 responsible guard dogs in the Metropolitan Region of Belém-PA, using the Polymerase Chain Reaction method (PCR), developed by the Laboratory of Biomolecular Technology, of the Federal University of Pará. The frequency of infections in all years of study was 29.13% for E. canis, 15.30% for A. platys, and 9% for B. vogeli. In addition, there was an increase in infection by E. canis and A. platys over the years and a reduction in infection by B. vogeli. In the analysis of co-infections, the number of animals infected simultaneously by E. canis, and A. platys indicates an association between these bacteria, different from infection by B. vogeli. The present research developed in the Metropolitan Region of Belém indicates that studies of the population dynamics of the vector tick must be carried out in the city so that control measures can be established to combat hemoparasitosis caused mainly by E. canis and A. platys, which has increased significantly each year and due to its zoonotic character.
Palavras-chave:E. canis; A. platys; B. vogeli; Molecular Diagnostic; Eastern Amazon.
1 INTRODUÇÃO
Hemoparasitosis is a disease caused by bacteria or obligatory intracellular protozoa that infect vertebrate blood cells. They transmit blood-sucking arthropods such as fleas and ticks, which transmit bioagents through saliva during blood feeding, infecting animals. Among the most studied hemoparasitosis are those caused by the bioagents of the genera Babesia, Ehrlichia, and Anaplasma, causing diseases that are responsible for high morbidity among pets and great economic losses, as they affect various animal species such as dogs and cats, horses and cattle (Ismail et al., 2010). The importance of hemoparasitosis is not only due to its pathogenic character among domestic species, such as canines but also to the zoonotic character of these diseases, requiring the clinical treatment of infected animals and sanitary cleaning of the places where these animals reside, aiming at elimination of vectors and decreasing the risk of human contamination (Figueiredo, 2011). Before blood smear tests made the diagnosis, the decision to use the antibiotic was often based only on signs compatible with the disease and the occurrence of the tick in the animal. In Belém, the diagnosis by molecular methods, such as PCR, became available to the community in 2013 through the extension service of the Biomolecular Technology Laboratory (BTL).
2. MATERIALS AND METHODS
2.1 TYPE OF STUDY
The retrospective study was developed by analyzing the records that are part of the diagnostic service’s collection of results for infections by hemoparasites in dogs at the Biomolecular Technology Laboratory of the Institute of Biological Sciences (ICB), Federal University of Pará.
2.2 CASUISTIC (STUDY POPULATION)
The study’s target population was composed of responsible guard dogs sent by veterinary clinics and hospitals in the metropolitan region of Belém from 2013 to 2017 (N=6.593 animals).
2.3 DATA COLLECT
The presence of E. canis and A. platys in the samples was evaluated based on the nested PCR protocol described by Rufino et al. (2013b). Canine babesiosis was diagnosed based on an optimized semi-nested PCR protocol, which partially amplifies the 18S rDNA gene from B. vogeli, according to Moraes et al. (2014).
2.4 Selection criteria
Inclusion criteria: Animals with records of sex, age, and race information in the database; Animals from the metropolitan region of Belém
Exclusion criteria: Animals with no record of sex and/or age and/or race; Animals from municipalities outside the metropolitan region of Belém; Animals who performed post-treatment exams for hemoparasitosis.
2.5. HYPOTHESIS
The prevalence of E. canis, B. vogeli, and A. platys remains constant or fluctuates over the years.
2.6 STATISTICAL ANALYSIS
The compiled results were analyzed statistically using the Qui-square (x2) test, with Yates correction, using the statistical program Bioestat 5.0.
3. RESULTS
3.1 SAMPLE CHARACTERIZATION
A total of 6.593 animals that underwent molecular testing (PCR) to detect the DNA of hemoparasites E. canis, B. vogeli, and A. platys were evaluated from the LTB database from 2013 to 2017. Of these animals, 3,399 (51.55%) were male, 3.194 (48.45%) were female, 1,444 (21.9%) were animals with up to one year of age, 3.164 (47.9%) were animals from one to seven years old and 1.985 (30.2%) were over seven years old.
3.2 frequency of hemoparasites E. canis, A. platys, and B. vogeli in the years 2013 to 2017
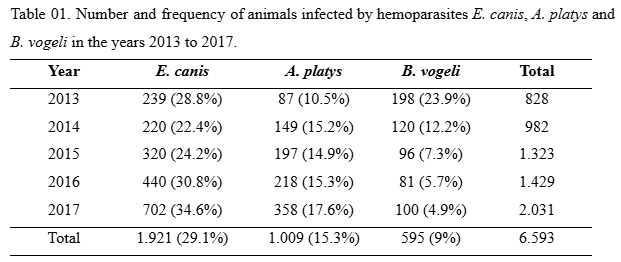
In table 01, it is possible to see the different frequencies over the years of study for hemoparasites E. canis, A. platys and B. vogeli, respectively. After analysis, it was possible to observe that the frequency of infection by E. canis was higher in 2016 and 2017, with percentages of 30.8% and 34.6%, respectively. Regarding the frequency of infection by A. platys, there was an increase from the year 2014 (15.2%) and remained relatively stable during the years 2015 (14.9%) and 2016 (15.3%). In 2017, this frequency increased slightly, reaching 17.6% of infected animals. In the analysis of animals infected with B. vogeli, the reduction in the frequency of cases throughout each year was evident. It can be observed that it decreased by 50% in 2014 (12.2%) from that recorded in 2013 (23.9%) and continued to decrease in subsequent years, with a frequency of 7.3% in 2015, 5.7% in 2016 and 4.9% in 2017.To assess whether the increase or reduction of infections by the three hemoparasites, the cui-square test (χ2) was performed, which showed that there was a significant increase in the frequency of infection by E. canis (χ2=38,04), B. vogeli (χ2= 234,21) and reduction in the frequency of infection by A. platys (χ2=18,60). Results with p<0.001 were significant.
3.3 OCCURRENCE OF CO-INFECTION AMONG THE HEMOPARASITES STUDIED
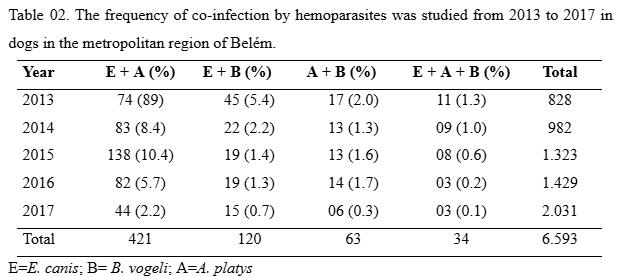
After analysing the frequency of blood parasites in animal samples, an analysis of mixed infections was performed among the target blood parasites in the present study. Co-infection was identified among the hemoparasites studied from 2013 to 2017, with a frequency of 9.6% (638/6.593) of the total animals analysed. In all associations (N=638) analysed in this study, it was possible to highlight the presence of E. cannis hemoparasite in ¾ of the mixed infections associated with one or more hemoparasites.Mixed infection between E. cannis and A. platys hemoparasites was more frequent in all years analysed in the present study than other observed co-infections. These two hemoparasites were more frequent in samples from the year 2015, with the presence in 10.4% (138/1,323) of animal samples (Table 02), with a p-value <0.001. Co-infection by E. canis and A. platys was relatively higher in all years analysed, among the samples of the years evaluated in the study when compared to the analysis of other co-infection of all years studied (p<0.001).
DISCUSSION
Infection by different hemoparasites is a constant and can be caused by different species of bacteria and protozoa, which infect the blood cells of the vertebrate host. Its transmitting agents are fleas and ticks that transmit a certain disease through the inoculation of the agent through saliva during the blood meal (Ismail et al., 2010).
Several species can cause hemoparasitosis in animals, the most studied being those caused by Ehrlichia canis, Anaplasma platys, and Babesia vogeli. Thus, the aim of this study was to verify the prevalence of these hemoparasites in dogs domiciled in the metropolitan region of Belém during the years 2013 to 2017. An analysis was made of the results of PCR tests located in the Biomolecular Technology Laboratory database at UFPa.
Conventional PCR is considered a gold diagnostic method, as it has high sensitivity and specificity compared to conventional parasitological tests, which occasionally can lead to false negative results, with good efficacy in the diagnosis of many parasitic diseases (Nakaghi et al., 2008; Lauerman, 1998).
The observed frequency of E. canis was the highest among the hemoparasitosis studied, ranging from 28.8% to 34.6% in animals. Our results are in agreement with studies conducted in Brazil that presented a frequency ranging from 18% to 53%, which also used PCR as a diagnostic method. (Dagnone et al., 2003; Bulla et al., 2004; Macieira et al., 2005; Santos et al., 2009; Nakaghi at al., 2008).
Rufino et al. (2013) evaluated samples of dogs from the city of Belém (Pa) treated at the Veterinary Hospital of the Federal Rural University of Amazônia and found E. canis infection in 24% of the dogs, which were randomly selected. Other studies that also used PCR showed similar results, such as those of Ramos et al. (2010) in Recife, which showed 38.04% of dogs with clinical suspicion were infected with the hemoparasite. Ueno et al. (2009) evaluated the infection in dogs that presented clinical signs suggestive of ehrlichiosis, treated at the Veterinary Hospital of Botucatu (SP) and found the parasite’s DNA in 40% of the analyzed samples.
Another data analyzed in the study was the frequency of A. platys infection, which ranged from 10.5% to 17.6% in different years. Other research using PCR found a very varied frequency, some of them being similar to those found in this study, such as that carried out by Ferreira et al. (2007) in Rio de Janeiro, with 15.8% of infected animals. Lasta (2011), in Porto Alegre, obtained a frequency of 13.56% in animals, while Costa Jr. (2007), in Minas Gerais, observed a frequency of 11.69%. Lower prevalences were described by Costa-Júnior et al. (2013) in Belo Horizonte (MG), with 5% of asymptomatic dogs with A. platys, in Goiás where the prevalence described by Costa (2015) was 4.5%, and Krause et al. (2015) recorded a frequency of only 1.1% of infection in dogs with suggestive symptoms in Pelotas (RS). The highest frequency was described by Ramos et al. (2010) in Recife, who found 48.78% of infection in animals with clinical suspicion of the disease. In Piauí, 41.5% of the animals that were randomly selected in Clinics and the Veterinary Hospital were infected by A. platys (Silva, 2010). Souza et al. (2004) in Campo Grande (MS) found a frequency of 41.9% in dogs with suggestive symptoms.
In relation to the species B. vogeli, it was shown that the frequency of infection by the parasite decreased during the years of the study, with a higher occurrence in 2013 with 24% of positive animals and lower in 2017, with only 5% of the dogs studied infected. Our results are similar to studies carried out using PCR as a diagnosis in Recife, where Ramos et al. (2010) found that 7.31% of the study dogs were infected with B. vogeli while Ribeiro (2017) found a frequency of 10.9% in dogs not domiciled in Paraná and Vieira (2017) analyzing dogs domiciled in Espírito Santo (ES), with a history of ticks, obtained a frequency of only 1.3%.
Analyzing the frequency variation over the years, it could be observed that infection by E. canis and A. platys is increasing significantly. Vector distribution studies in the city and metropolitan region of Belém are not available. Thus, there are no public policies to control the tick in the city, which seems to be helping its maintenance and reproduction, as it has ideal weather conditions to occur in abundance. This factor, associated with the total lack of knowledge about resistant strains, seems to be contributing to the lack of control of the infection of these bacteria.
The number of infected animals is directly associated with vector distribution (Rodrigues-Vivas et al., 2005; Carlos et al., 2007). Studies on the dynamics of the Riphicephalus sanguineus tick, such as its distribution in the environment, prevalence, and form as the climate affects, these factors are extremely important, as they can affect its transmission cycle and possibly also alter the frequency of infections (Kovass et al., 2001). There are still no studies on Riphicephalus sanguineus in Pará, and it is not possible to make comparisons and associations with the frequencies of infections in the study.
Another important fact observed was the presence of simultaneous infection among hemoparasites in all years of the study. The most frequent co-infection was by the bacteria E. canis and A. platys. Other studies have already reported the occurrence of co-infections among the parasites analyzed in this study, such as Silva et al. (2012), Ribeiro et al. (2017), Santos et al. (2009), Ramos et al. (2009). The analysis showed that the occurrence of dogs co-infected with E. canis and A. platys is significantly higher than the co-infection with one of the bacteria and the protozoan B. vogeli.
These data indicate that the coexistence of E. canis and A. platys in the vector tick, which belongs to the same family (Anaplasmataceae), may be facilitated by a symbiotic interaction between them (Moutailler et al., 2016). On the other hand, infection by B. vogeli seems to occur preferentially in an isolated way. In the present study, co-infection of B. vogeli with the study bacteria was not observed, which does not promote a harmonic relationship, indicating competition within the tick vector.
In co-infections, one of the pathogens can act as a facilitating agent for other infections to establish themselves, which can aggravate clinical signs and also intensify immunosuppression, making the parasitized animals more prone to high parasitemia (Sousa, 2012), in addition to aggravating the animal’s clinical condition, as indicated by Santos et al. (2009) who found significant values of thrombocytopenia especially when there was co-infection between E. canis and A. platys. Gaunt et al.(2010) demonstrated in experimentally infected dogs that simultaneous infection by E. canis and A. platys can impact hematological changes, alter the immune response, aggravate clinical signs and can cause a more persistent infection by A. platys.
In the current study, there was an increase in the frequency of infection by E. canis and A. platys over the years, serving as a warning to the importance of applying prophylactic measures for the disease. The increase in frequency may be associated with the presence of the vector tick in the city, which has the ideal climatic conditions for its proliferation, such as humidity and temperature, in addition to the presence of strains resistant to the antibiotic commonly used to treat the disease.
The confirmation of the diagnosis of the disease in Belém has been carried out for a few years through PCR, which may have influenced the indiscriminate use of antibiotics in previous years, which could have led to the strains becoming resistant to the drug. With this, the study contributes in a relevant way in supporting the development of public policies that help in the control of these infections, which have a zoonotic character.
CONCLUSIONS
After analyzing the data, it was possible to conclude that:
The frequency of infection by E. canis, A. platys, and B. vogeli was observed in the years 2013 to 2017 in the metropolitan region of Belém after molecular diagnosis by PCR;
Infection with E. canis and A. platys increased significantly during the last years of the study;
Co-infection was observed in all years of the study, being significantly higher among hemiparasites E. canis and A. platys;
There was no correlation between infection by these hemoparasites and the variables sex, age and breed of animals, as the infection depends on the presence of the vector.
REFERÊNCIAS
ISMAIL N, BLOCH KC, MCBRIDE JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010 Mar;30(1):261-92. doi: 10.1016/j.cll.2009.10.004.
FIGUEIREDO MR. Babesiose e erliquiose caninas. Monografia (Especialização em Clínica Médica de Pequenos Animais). Rio de Janeiro, 2011. Disponível em https://pt.scribd.com/document/343544772/Babesiose-e-Erliquiose-Monica-Ramos-Figueiredo-pdf.
RUFINO CP, MORAES PH, REIS T, CAMPOS R, AGUIAR DC, MCCULLOCH JA, MENESES AM, GONÇALVES EC. Detection of Ehrlichia canis and Anaplasma platys DNA using multiplex PCR. Vector Borne Zoonotic Dis. 2013 Dec;13(12):846-50. doi: 10.1089/vbz.2013.1303.
MORAES PHG, RUFINO CPRT, AGUIAR DCF, MENESES AMC, GONÇALVES EC. Optimization of a molecular method for the diagnosis of canine babesiosis. Revista Brasileira de Parasitologia Veterinária, v. 23, n. 1, p. 105-108, 2014. doi: 10.1590/s1984-29612014017.
NAKAGHI ACH, MACHADO RM, COSTA MT, ANDRÉ MR, BALDANI CD. NAKAGHI A. Canine ehrlichiosis: clinical, hematological, serological and molecular aspects. Ciência Rural, v. 38, n. 3, pág. 776-770, 2008. https://doi.org/10.1590/S0103-84782008000300027.
LAUERMAN LH. Nucleic acid amplification assays for diagnosis of animal diseases. Washington: Ed Library of Congress Cataloging-in- Publication Data. 1998. 152 p.
MARTIN AR, BROWN GK, DUNSTAN RH, ROBERTS TK. Anaplasma platys: an improved PCR for its detection in dogs. Exp Parasitol. 2005 Mar;109(3):176-80. doi: 10.1016/j.exppara.2004.11.007.
GASSER RB. Molecular tools—advances, opportunities and prospects. Veterinary Parasitology, 136:69-89, 2006. doi: 10.1016/j.vetpar.2005.12.002.
MOURA ST, FERNANDES CGN, RUFFINO S, SILVA VL, OLIVEIRA-JÚNIOR, PA. Ocorrência de hemoparasitos em cães de Cuiabá·, estado de Mato Grosso. In: XII Congresso Brasileiro de Parasitologia Veterinária, 2002, Rio de Janeiro. Rio de Janeiro, 2002.
SOUZA, AI, DAGNONE AS, MACHADO RZ. Infecção por Anaplasma platys em cães de Campo Grande, MS. Rev. Bras. Parasitol. Vet., 13 (Suppl. 1) 2004, p. 352.
DAGNONE AS, MORAIS HSA, VIDOTTO MC, JOJIMA FS, VIDOTTO O. Ehrlichiosis in anemic, thrombocytopenic, or tick-infested dogs from a hospital population in South Brazil. Vet Parasitol. 2003 Nov 28;117(4):285-90. doi: 10.1016/j.vetpar.2003.10.001.
BULLA C, TAKAHIRA RK, ARAÚJO JRJP, TRINCA LA, LOPES RS, WIEDMEYER CE. Bulla C, The relationship between the degree of thrombocytopenia and infection with Ehrlichia canis in an endemic area. Vet Res. 2004 Jan-Feb;35(1):141-6. doi: 10.1051/vetres:2003038.
MACIEIRA DB, MESSICK JB, CERQUEIRA AM, FREIRE IM, LINHARES GF, ALMEIDA NK, ALMOSNY NR. Prevalence of Ehrlichia canis infection in thrombocytopenic dogs from Rio de Janeiro, Brazil. Vet Clin Pathol. 2005;34(1):44-8. doi: 10.1111/j.1939-165x.2005.tb00008.x.
SANTOS F, COPPEDE JS, PEREIRA AL, OLIVEIRA LP, ROBERTO PG, BENEDETTI RB, ZUCOLOTO LB, LUCAS F, SOBREIRA L, MARINS M. Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirão Preto, Brazil. Vet J. 2009 Jan;179(1):145-8. doi: 10.1016/j.tvjl.2007.08.017.
RAMOS R, RAMOS C, ARAÚJO F, OLIVEIRA R, SOUZA I, PIMENTEL D, GALINDO M, SANTANA M, ROSAS E, FAUSTINO M, ALVES L. Molecular survey and genetic characterization of tick-borne pathogens in dogs in metropolitan Recife (north-eastern Brazil). Parasitol Res. 2010 Oct;107(5):1115-20. doi: 10.1007/s00436-010-1979-7.
UENO TEH, AGUIAR DM, PACHECO RC, RICHTZENHAIN LJ, RIBEIRO MG, PAES AC, MEGID J, LABRUNA MB. Ehrlichia canis em cães atendidos em hospital veterinário de Botucatu, Estado de São Paulo, Brasil. Revista Brasileira de Parasitologia Veterinária, Jaboticabal, v. 18, n. 3, p. 57-61, 2009. https://doi.org/10.4322/rbpv.01803010.
COSTA-JÚNIOR LM, REMBECK K, PASSOS LM, RIBEIRO MF. Factors associated with epidemiology of Anaplasma platys in dogs in rural and urban areas of Minas Gerais State, Brazil. Prev Vet Med. 2013 May 1;109(3-4):321-6. doi: 10.1016/j.prevetmed.2012.10.011.
CARVALHO FS, WENCESLAU AA, CARLOS RS, ALBUQUERQUE GR. Epidemiological and molecular study of Ehrlichia canis in dogs in Bahia, Brazil. Genet Mol Res. 2008 Jul 29;7(3):657-62. doi: 10.4238/vol7-3gmr468.
FERREIRA R.F., CERQUEIRA A.M.F., PEREIRA A.M., GUIMARÃES C.M., SÁ A.G., ABREU F.S., MASSARD C.L. & ALMOSNY N.R.P. 2007. Anaplasma platys diagnosis in dogs: comparison between morphological and molecular tests. Intern. J. Appl. Res. Vet. Med. 5:113-119.
LASTA, C.S. Fatores de risco, parâmetros hematológicos, detecção molecular e sorológica de Erlichia canis e Anaplasma platys em cães de Porto Alegre/RS. Dissertação (Mestrado) –Universidade Federal do Rio Grande do Sul. Faculdade de Veterinária. Programa de Pós-graduação em Ciências Veterinárias., 2011. Disponível em https://lume.ufrgs.br/handle/10183/117124.
COSTA-JÚNIOR LM, REMBECK K, PASSOS LM, RIBEIRO MF. Factors associated with epidemiology of Anaplasma platys in dogs in rural and urban areas of Minas Gerais State, Brazil. Prev Vet Med. 2013 May 1;109(3-4):321-6. doi: 10.1016/j.prevetmed.2012.10.011.
COSTA HX. Anaplasma platys e Ehrlichia canis em cães: Avaliação de alterações oculares, desenvolvimento e validação de técnica de diagnóstico molecular. Tese (Doutorado em Ciência Animal) – Escola de Veterinária e Zootecnia da Universidade Federal de Goiás, Goiás, 2015. Disponível em: https://repositorio.bc.ufg.br/tede/items/68a935d7-abc7-4ad3-b62d-fc8c5576c606.
KRAUSE, L. S., RIBEIRO, L., IÉCK, C., FARIAS, B., RUAS, N. Molecular characterization of Anaplasma platys in dogs in Pelotas city, Southern Brazil. Scholars Journal of Agriculture and Veterinary Sciences. 3. 20-25, 2016. DOI: 10.36347/sjavs.2016.v03i01.003.
SILVA JN, ALMEIDA ADO B, BOA SORTE EDA C, FREITAS AG, SANTOS LG, AGUIAR DM, SOUSA VR. Seroprevalence anti-Ehrlichia canis antibodies in dogs of Cuiabá, Mato Grosso. Rev Bras Parasitol Vet. 2010 Apr-Jun;19(2):108-11. Portuguese. doi: 10.4322/rbpv.01902008.
RIBEIRO CM, MATOS AC, AZZOLINI T, BONES ER, WASNIESKI EA, RICHINIPEREIRA VB, VIDOTTO O. Molecular epidemiology of Anaplasma platys, Ehrlichia canis and Babesia vogeli in stray dogs in Paraná, Brazil. SMALL ANIMAL DISEASES Pesq Vet Bras. 37 (02) Feb 2017 https://doi.org/10.1590/S0100-736X2017000200006.
VIEIRA FT. Ocorrência de Ehrlichia spp., Anaplasma spp., Babesia spp., Hepatozoon spp. e Rickettsia spp. Em cães domiciliados em seis municípios do Estado do Espírito Santo, Brasil. Tese (Doutorado em Doenças Infecciosas) – Núcleo de Doenças Infecciosas, Universidade Federal do Espírito Santo, Espírito Santo. 2017. Disponível em https://dspace4.ufes.br/items/403b0caf-abf5-46d9-b0b7-fc3390912afb/full.
SILVA WAC. Ocorrência da infecção por Ehrlichia spp e Anaplasma platys em canídeos e felídeos selvagens mantidos em cativeiro no Distrito Federal e Goiás. Dissertação (Mestrado em Saúde Animal) – Universidade de Brasília. 2016. Disponivel em: http://educapes.capes.gov.br/handle/capes/880470.
BRAGA JFV. Babesiose canina em Teresina, Piauí. Dissertação (Mestrado em Ciência Animal) – Universidade Federal do Piauí, Teresina, 60p 2011.
SPOLIDORIO MG, MINERVINO AH, VALADAS SY, SOARES HS, NEVES KA, LABRUNA MB, RIBEIRO MF, GENNARI SM. Serosurvey for tick-borne diseases in dogs from the Eastern Amazon, Brazil. Rev Bras Parasitol Vet. 2013 Apr-Jun;22(2):214-9. doi: 10.1590/S1984-29612013005000023.
TRAPP SM, MESSICK JB, VIDOTTO O, JOJIMA FS, DE MORAIS HS. Babesia gibsoni genotype Asia in dogs from Brazil. Vet Parasitol. 2006 Oct 10;141(1-2):177-80. doi: 10.1016/j.vetpar.2006.04.036.
FONSECA, J. P., BRUHN, F. R. PASCOTI, R., MACIEL, M. J., HIRSCH, C., MAGALHÃES, C. M. B., GUEDES, E. & GUIMARÃES, A. M. Hematological parameters and seroprevalence of Ehrlichia canis and Babesia vogeli in dogs. Ciênc. anim. bras. 18 2017 https://doi.org/10.1590/1089-6891v18e-36095.
RODRIGUEZ-VIVAS RI, ALBORNOZ RE, BOLIO GM. Ehrlichia canis in dogs in Yucatan, Mexico: seroprevalence, prevalence of infection and associated factors. Vet Parasitol. 2005 Jan 4;127(1):75-9. doi: 10.1016/j.vetpar.2004.08.022.
CARLOS RSA, MUNIZ-NETA ES, SPAGNOL FH, OLIVEIRA LLS, BRITO LLR, ALBUQUERQUE GR, ALMOSNY NRP. Frequência de anticorpos anti-Erhlichia canis, Borrelia burgdorferi e antígenos de Dirofilaria immitis em cães na microrregião Ilhéus-Itabuna, Bahia, Brasil. Revista Brasileira de Parasitologia Veterinária, Jaboticabal, v.16, n.3, p.117-120, 2007. https://doi.org/10.1590/S1984-29612007000300001.
KOVATS RS, CAMPBELL-LENDRUM DH, MCMICHAEL AJ, WOODWARD A, COX JS. Early effects of climate change: do they include changes in vector-borne disease? Philos Trans R Soc Lond B Biol Sci. 2001 Jul 29;356(1411):1057-68. doi: 10.1098/rstb2001.0894.
SILVA GCF, BENITREZ AN, GIROTTO A, TARODA A, VIDOTTO MC, GARCIA JL, FREITAS JC, HEADLEY SA, VIDOTTO, O. Occurrence of Ehrlichia canis and Anaplasma platys in household dogs from northern Parana. Revista Brasileira de Parasitologia Veterinária, v. 21, n. 4, p. 379-385, 2012. https://doi.org/10.1590/S1984-29612012005000009.
RAMOS R, RAMOS C, ARAÚJO F, OLIVEIRA R, SOUZA I, PIMENTEL D, GALINDO M, SANTANA M, ROSAS E, FAUSTINO M, ALVES L. Molecular survey and genetic characterization of tick-borne pathogens in dogs in metropolitan Recife (north-eastern Brazil). Parasitol Res. 2010 Oct;107(5):1115-20. doi: 10.1007/s00436-010-1979-7.
MOUTAILLER S, VALIENTE MORO C, VAUMOURIN E, MICHELET L, TRAN FH, DEVILLERS E, COSSON JF, GASQUI P, VAN VT, MAVINGUI P, VOURC’H G, VAYSSIER-TAUSSAT M. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl Trop Dis. 2016 Mar 17;10(3):e0004539. doi: 10.1371/journal.pntd.0004539.
SOUSA KCM. Co-infecção por Ehrlichia canis, Leishmania chagasi e Babesia canis em cães naturalmente infectados em Campo Grande, Mato Grosso do Sul. Dissertação (Mestrado em Medicina Vetrinária) – Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias, 88 f.2012. Disponível em https://repositorio.unesp.br/entities/publication/639f844f-4781-4ae7-9681-200cd1873c47.
SANTOS F, COPPEDE JS, PEREIRA AL, OLIVEIRA LP, ROBERTO PG, BENEDETTI RB, ZUCOLOTO LB, LUCAS F, SOBREIRA L, MARINS M. Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirão Preto, Brazil. Vet J. 2009 Jan;179(1):145-8. doi: 10.1016/j.tvjl.2007.08.017.
VAN HEERDEN J, REYERS F, STEWART CG. Van Heerden J, Reyers F, Stewart CG. Treatment and thrombocyte levels in experimentally induced canine ehrlichiosis and canine babesiosis. Onderstepoort J Vet Res. 1983 Dec;50(4):267-70.
GAUNT S, BEALL M, STILLMAN B, LORENTZEN L, DINIZ P, CHANDRASHEKAR R, BREITSCHWERDT E. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors. 2010 Apr 8;3(1):33. doi: 10.1186/1756-3305-3-33.
1Bióloga e Mestre em Biologia de Agentes Infecciosos e Parasitários pela Universidade Federal do Pará;
2Médica Veterinária e Doutora em Biologia de Agentes Infecciosos e Parasitários pela Universidade Federal do Pará;
3Biólogo e Doutor em Biotecnologia pela Universidade Federal do Pará;
4Médico Veterinário e Doutora em Biologia de Agentes Infecciosos e Parasitários pela Universidade Federal do Pará;
5Docentes do Instituto de Ciências Biológicas da Universidade Federal do Pará
