REGISTRO DOI: 10.69849/revistaft/th102411131043
Eduardo José Andrade Lopes
Augusto Paraíso Martins Neto
Fernanda Oliveira de Andrade Lopes
Victor Rivera Silveira Barretto
Carlos André Balthazar
Paula Oliveira de Andrade Lopes
André Pessoa Bonfim Guimarães
Introduction
Patients frequently seek penile enlargement and girth enhancement procedures at the urologist’s office. New surgical and non-surgical techniques have emerged in recent years, creating a promising horizon in an area with a lot to evolve. The advent of hyaluronic acid (HA) with higher viscosity, high grammage and slow absorption1, has enabled possible treatments with greater safety and better results. Aesthetic and non-aesthetic situations, such as when the patient has a penis size where no condom remains in the organ during sexual activity can be solved with penile enlargement.
Since 2011, doctors in Asian countries such as South Korea, Japan and China have been publishing about penile girth enhancement techniques with HA1–5. Understanding the psychological impact of concerns about penis size is crucial for addressing the well-being of individuals. Penises with diameters at rest below 11 cm and lengths less than 12 cm are directly related to Small Penis Syndrome (SPS)6. Body insecurity and dissatisfaction, particularly when the penis is at rest, can contribute to erectile dysfunction as a consequence of the established psychological disorder, often unresponsive to psychological treatment. SPS is a syndrome that results from the anxiety felt when someone’s penis is being observed, driven by the concern that the flaccid penis is smaller than the ideal size for an adult man7. SPS can progress or be the underlying disease to a more serious situation characterized in the literature as corporal dysmorphism disorder (CDD)7,8, and evento penile dysmorphic disorder (PDD)7–9. Situation that determines an incessant search for treatment and that requires psychological and psychiatric support, in most cases.
In Asian countries, such as Korea, there are temporary fillers with HA, exclusively for use in penile thickening2–4,9. In the United States, HA has been approved as filler for human use since 2003. Meanwhile, in Brazil, the National Health Surveillance Agency (ANVISA) not only authorizes the use of HA, but also polymethylmethacrylate (PMMA) and polylactic acid (PLA) for deep application (intramuscular and above the bone) as well as the subcutaneous region.ANVISA provides specific guidelines for appropriate weights and percentages for each location on the human body, including 30%, 10%, 5% and 2% for PMMA, and 20 and 30 mg, for both HA, and PLA10. PMMA, HA and PLA are frequently used in various specialties with low complication rates (<1%), particularly in the gluteal region11 and in penis enlargement10,12,13 to promote increased volume.
SPS, micropenis and amputated penis are surgically treated with the use of autologous tissues including corpora cavernosa, de-epithelized foreskin and scrotum, dartos and vaginal layer with the intention of promoting thickening and lengthening14,15. However, non-surgical thickening with temporary products has grown exponentially in the last five years with the production of HA and PLA with higher grammage, higher viscosity, slow absorption, more accessible prices, and notably improved application techniques5,14–16. We aimed to analyze our results using hyaluronic acid for penis enlargement.
Material and Methods
All research procedures adhered to CNS Resolution No. 466/2012, the Declaration of Helsinki, and principles of ethics including non-maleficence, beneficence, and autonomy. Patients were assured the right to withdraw from the study at any time, and their privacy and confidentiality were protected.
Sample selection and intervention
Patients who attended private service during the period of March 2018 and February 2019 with complaints associated with penis size were selected. All of them were treated with HA infiltration and followed up prospectively for one year. We used HA with a weight of 30 mg, high viscosity, injected with a stainless-steel cannula, graduated and lateral holes, 15 cm long and 13 French in diameter, through a single orifice in the pubic region, followed by blunt dissection – back and forth -, and manual molding.
Penis filling technique
The primary author administered the filler comprising 30 mg HA undiluted. The infiltrations were performed in an outpatient minor surgery unit under local anesthesia. In cases where the clinical condition warranted additional care, the procedures were conducted in a day hospital.
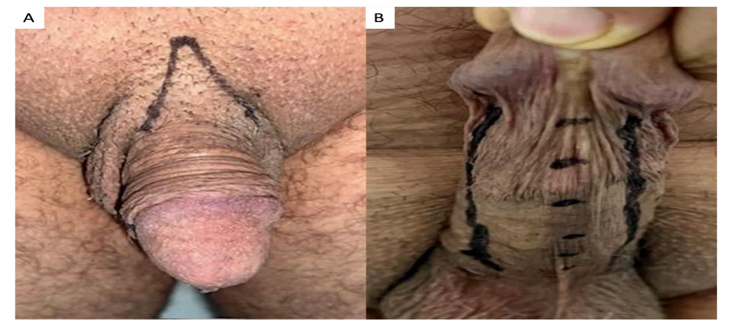
In a hospital setting, patients underwent intravenous sedation performed by a qualified anesthesiologist. To ensure precision during the procedure, meticulous dorsal (Fig. 1A) and ventral (Fig. 1B) markings were performed to guide the path of the cannula. Ventral markings specifically targeted the spaces on both sides of the urethra, outlined with a distinct solid line (Fig. 1B). The urethral region was marked by points spaced at intervals of approximately half a centimeter. An orifice was created in the dorsal (anterior) region, precisely two centimeters away from the penis, and in the pubic region (Fig. 1A e 1B).
Figure 1: A) Local anesthesia and cannula entry hole. B) Visual marking of the urethra and lateral spaces to be filled.
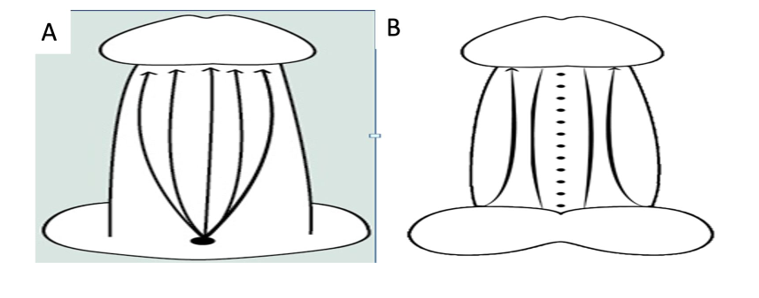
The technique involved utilizing our specialized cannula (BUC) with a blunt tip and flexible shaft to perform the divulsion and detachment of the skin and create a space. Executing forward movements towards the glans, and backwards to approach the pubic region, all beneath the skin layer and within the deep fascia, seeking to preserve vessels and nerves (Figs. 2A and 2B).
Figure 2A and B: Dorsal and ventral access.
To allow the passage of the cannula 13, it was necessary to make a small cut with the tip of the scalpel blade 11. Employing careful back-and-forth motions, the cannula was navigated through the incision, threading its way through the deep fascia, providing greater distribution of the filler injected by retro injection. HA deposition was carried out, in all cases, through a single dorsal (anterior) port, rotating the penis to initially fill the right ventral region, followed by the left ventral region – maintaining a parallel trajectory to the urethra.
Following closely as the second most common complaint, was the dissatisfaction with the perceived small size of the organ at rest, causing behavioral changes, such as avoiding wearing beach shorts, refraining from exposing themselves naked around others, and limiting sexual activities to low-light environments. In response to these concerns, we proposed HA infiltration using specially developed cannulas, derived from products approved by ANVISA10, using a single dorsal port (orifice) in the pubic region, and was performed under local anesthesia (Figs. 1A and 1B). The HA that was injected into the penis is used by plastic surgeons and dermatologists diluted in a 0.9% saline solution in a 50/50% ratio. With this dilution it is possible to inject using an 18 French (Fr) cannula. Unfortunately, the absorption is faster at around 6 months in those cases. Therefore, to be able to inject without diluting and increase absorption time, we developed a stainless-steel cannula, 15 cm long and 13 fr in diameter. With this diameter, blunt tip (non-perforating), and side holes (being the two largest at the distal end), we were able to inject the HA without dilution.
Following filler application, the penis was thoroughly massaged to ensure the even distribution of the HA. To safeguard against infection, all patients received prophylactic antibiotic therapy, initiated two hours before the procedure and continued for another 48 hours. To reduce the edema, 20 mg of corticosteroids were administered and continued for another five days, along with analgesic use. Patients were advised to abstain from sexual intercourse or masturbation for a period of 15 days.
Outcomes and data collection
Penile circumferences were measured and documented in a state of total flaccidity. We used HA with a weight of 30 mg, high viscosity, injected with a stainless-steel cannula, graduated and lateral holes, 15 cm long and 13 French in diameter, through a single orifice in the pubic region, followed by blunt dissection – back and forth -, and manual molding. A measuring tape was used with the penis at rest, placed in the middle third, 2.0 cm from the balano-preputial groove. We adopted and standardized a single measurement taking as a reference the studies by Yang2–4who found no statistically significant mean difference when measuring in three locations on the penis. Patients with a diameter of 7.0 to 8.0 cm reported the involuntary displacement of condoms during sexual intercourse.
Changes in penile circumference were measured with a flexible measuring tape, two centimeters below the lower edge of the glans, before (Dp0), immediate post-filling (Dp1), after 01 month (2), and after 12 months (Dp3). The patient’s sexual satisfaction was assessed by the patient’s own subjective perception in percentage terms regarding sexual frequency, and the time to ejaculate compared to the situation prior to the procedure.
Sexual performance was assessed subjectively by three questions: 1) By what percentage did the frequency of your sexual activity increase compared to what it was before the procedure? 2) What percentage did the penile girth enhancement increase your self-esteem? 3) The time to ejaculate increased by what percentage?The average percentage obtained was defined as Sexual Satisfaction after Penis Filling (SSpF).
Statistical analysis
Categorical data were presented as absolute counts (n) and relative frequency (%). Contingency matrices were analyzed using Pearson’s Chi-square test, while complex matrices (e.g., 2×3, 3×4) were partitioned into simpler 2×2 matrices for improved determination of causality.
The continuous and semi-continuous data of the variables were compared using the Gaussian distribution curve, determined as non-parametric through the K-S Distance test (Kolmogorov-Smirnov), and the Shapiro-Wilk test. Hence, they were represented by median and percentiles (25th – 75th). When comparing two independent groups, the Mann-Whitney test with Bonferroni correction was employed.
For the entire study, an Alpha risk of less than or equal to 5% of committing a Type I or 1st species error was adopted, along with a Beta risk of less than or equal to 20% for committing a Type II or 2nd species error.
Results
Penile circumferences were measured and documented in a state of total flaccidity in fifty-three patients who attended private service during the period of March 2018 and February 2019 with complaints associated with penis size. The patients’ ages ranged from 24 to 67, average of 39.72 years. All of them were treated with HA infiltration and followed up prospectively for one year.
The median injected volume of 33.15 ml (20-40 ml) resulted in a significant increase in diameter, with the baseline circumference (Dp0) of 10.18 ± 1.11 cm expanding to the mean of 14.01 ± 1.13 (Dp1), finally averaging 14.2 ± 1.12 cm after 1 month (Dp2) (p < 0.001) and maintaining at 13,08 ± 1.14 cm up to 12 months (Dp3) (Table 01 and 02), (Fig 3A and 3B).
Tables
Table 1: Descriptive Statistics of the Sample
| N | Minimum | Maximum | Mean | Standard Deviation | |||
| Dp0 | 53 | 7 | 12.1 | 10.181 | 1.112 | ||
| HAVi | 53 | 20 | 40 | 33.151 | 4.794 | ||
| Dp1 | 53 | 9.9 | 15.7 | 14.019 | 1.131 | ||
| Dp2 | 53 | 10.2 | 15.9 | 14.260 | 1.129 | ||
| Dp3 | 53 | 9.1 | 15.2 | 13.087 | 1.143 | ||
SD: Standard Deviation; HAVi: injected volume; Dp0: before HA; Dp1: immediately after HA, Dp2: 1 month after HA, Dp3: 1 year after.
| Table 2: Pairwise Method Comparisons | ||||||
| (I) GE | (J) GE | Mean diference (I-J) | Error | P value | 95% ConfidenceInterval | |
| Lower Limit | Upper Limit | |||||
| 1 | 2 | -3.838* | .097 | .000 | -4.104 | -3.571 |
| 3 | -4.079* | .095 | .000 | -4.339 | -3.820 | |
| 4 | -2.906* | .108 | .000 | -3.201 | -2.610 | |
| 2 | 1 | 3.838* | .097 | .000 | 3.571 | 4.104 |
| 3 | -.242* | .014 | .000 | -.280 | -.203 | |
| 4 | .932* | .054 | .000 | .783 | 1.081 | |
| 3 | 1 | 4.079* | .095 | .000 | 3.820 | 4.339 |
| 2 | .242* | .014 | .000 | .203 | .280 | |
| 4 | 1.174* | .054 | .000 | 1.024 | 1.323 | |
| 4 | 1 | 2.906* | .108 | .000 | 2.610 | 3.201 |
| 2 | -.932* | .054 | .000 | -1.081 | -.783 | |
| 3 | -1.174* | .054 | .000 | -1.323 | -1.024 | |
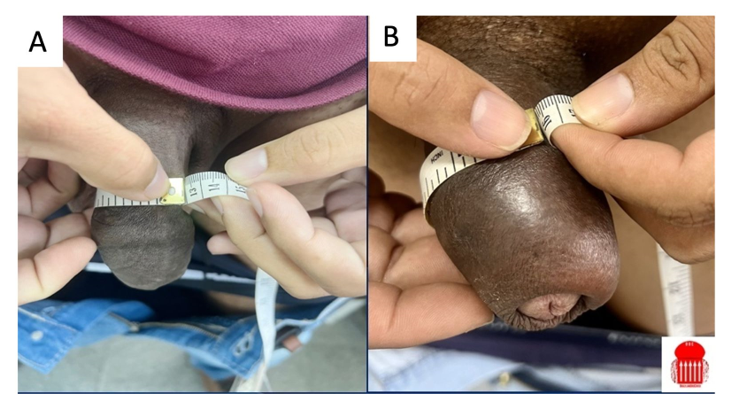
Figure 3: Filling with 40 ml of HA in a penis with foreskin: A) before (12 cm); B) after (16 cm).
Fig 3A and 3B:
All patients reported an increase in sexual performance assessed subjectively by the three questions on the SSpF, with a mean patient reported satisfaction was 82.64 ± 13.3. The satisfaction varied between 50 and 100%.
In three cases where partial absorption ofHA occurred after 8 months of follow-up, impacting performance, a 7.5 ml reinjection was administered to restore the initially achieved diameter; notably, these cases involved high-performance athletes and were subsequently excluded from the analysis. The study underscores a remarkable penile girth enhancement, showcasing its effectiveness persisting for an average duration of up to 12 months. Post-treatment, all patients experienced temporary edema, which subsided over the following days. Notably, only two cases of infection were encountered, promptly resolved with the administration of broad-spectrum antibiotics. Ecchymosis emerged as the most prevalent complication, spontaneously resolved within 15 days. Remarkably, there were no instances of bruising or inadvertent injections into vessels. The use of blunt tip cannula proved to be effective and safe. Noteworthy variations were observed in patients with excess foreskin.
The application of HA demonstrated successful thickening without major asymmetries. We encountered three cases characterized by the formation of “nodules” termed “bulking”, accompanied by the persistence of moderate edema, sensation of burning and itching on the skin requiring the use of corticosteroids for two weeks.
Discussion
Penis enlargement procedures are increasingly requested by men who are concerned about their penis size and/or appearance, especially in a flaccid state. Therefore, there is a great demand to develop safe, effective solutions and minimally invasive procedures to help. Enhancing penile girth using injectable HA fillers present a promising option, outperforming alternatives such as PMMA, and PLA, while offering a simpler solution than surgical procedures3,5,10–16. Though various filler products have been used over the years, each has its limitations and associated risks. To date, HA gels appear to be safe and effective, in addition to being absorbable in the medium term and presenting reversibility through hyaluronidase enzyme application. Although the existing scientific evidence remains elusive and the ideal product is yet to be identified, HA with increased weight, viscosity, and slow absorption emerges as a favorable choice, despite the high cost. Periodic applications are essential to maintain the desired penile diameter2,3,7,9. The current literature of high-quality studies with follow-up of two or more years, indicates that these applications should be repeated annually, and in smaller quantities than the initial approach2,3,7,9,10.
This research emphasizes the need for an aesthetic approach that minimizes the stigma of a small penis experienced by many men, promoting increased self-esteem, while at the same time signaling the need for the industry to seek the manufacture of a slower-absorbing HA, to reduce reapplication time.
While the absorbable nature of the product necessitates periodic reapplications, typically every 12 months, to sustain penile diameter—an easily manageable procedure performed safely under local anesthesia and on an outpatient basis—our study, albeit yielding promising outcomes, acknowledges the constraint of a modest sample size. With only 53 patients, a broader participant pool could offer more comprehensive insights. Moreover, the study underscores the necessity for further investigations to reinforce our conclusions, especially in addressing variations observed, such as the need for earlier reapplications at 8 months in two patients engaged in high-impact exercises.
Conclusions
In 2008 we initiated penis enlargement in selected cases, with autologous fat and polymethyl methacrylate (PMMA). Subsequently, starting from 2011 onwards we transitioned to utilizing HA. This article aims to present our technique and share the results of penile enlargement with HA.Our penile thickening technique using a specialized cannula and a single-entry port to fill the entire penile circumference, denominated as BUC (Brazilian Uro Cover), is effective in detaching and evenly distributing the product, while minimizing local complications. The consistently high patient satisfaction emphasizes the efficacy of this technique. Considering the limitations of the material, our technique methods for increasing penile circumference, despite the short follow-up and limited number of patients, proved to be very effective, safe and with consistent results. During the follow-up period, improvements in sexual activity and an increase in latency to ejaculate were reported. Penile thickening was achieved without significant asymmetries or complications.
Data availability statement
The data that support the findings of this study are available from the first author but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the first author.
References
1. Kwak T Il, Oh M, Kim JJ, Moon DG. The Effects of Penile Girth Enhancement using Injectable Hyaluronic Acid Gel, a Filler. J Sex Med. 2011;8(12):3407-3413. doi:10.1111/j.1743-6109.2010.01748.x
2. Yang DY, Ko K, Lee SH, Lee WK. A Comparison of the Efficacy and Safety Between Hyaluronic Acid and Polylactic Acid Filler Injection in Penile Augmentation: A Multicenter, Patient/Evaluator-Blinded, Randomized Trial. J Sex Med. 2019;16(4):577-585. doi:10.1016/j.jsxm.2019.01.310
3. Yang DY, Jeong HC, Ahn ST, et al. A Comparison Between Hyaluronic Acid and Polylactic Acid Filler Injections for Temporary Penile Augmentation in Patients with Small Penis Syndrome: A Multicenter, Patient/Evaluator-Blind, Comparative, Randomized Trial. J Sex Med. 2020;17(1):133-141. doi:10.1016/j.jsxm.2019.10.006
4. Yang DY, Jeong HC, Ko K, Lee SH, Lee YG, Lee WK. Comparison of clinical outcomes between hyaluronic and polylactic acid filler injections for penile augmentation in men reporting a small penis: A multicenter, patient-blinded/evaluator-blinded, non-inferiority, randomized comparative trial with 18 months of follow-up. J Clin Med. 2020;9(4):1-13. doi:10.3390/jcm9041024
5. Kim MT, Ko K, Lee WK, Kim SC, Yang DY. Long-Term Safety and Longevity of a Mixture of Polymethyl Methacrylate and Cross-Linked Dextran (Lipen-10®) after Penile Augmentation: Extension Study from Six to 18 Months of Follow-Up. World J Mens Health. 2015;33(3):202. doi:10.5534/wjmh.2015.33.3.202
6. Wylie KR, Eardley I. Penile size and the “small penis syndrome.” BJU Int. 2007;99(6):1449-1455. doi:10.1111/j.1464-410X.2007.06806.x
7. Veale D, Miles S, Read J, et al. Penile Dysmorphic Disorder: Development of a Screening Scale. Arch Sex Behav. 2015;44(8):2311-2321. doi:10.1007/s10508-015-0484-6
8. Sarwer DB, Spitzer JC. Body image dysmorphic disorder in persons who undergo aesthetic medical treatments. Aesthetic Surg J. 2012;32(8):999-1009. doi:10.1177/1090820X12462715
9. Dillon BE, Chama NB, Honig SC. Penile size and penile enlargement surgery: A review. Int J Impot Res. 2008;20(6):519-529. doi:10.1038/ijir.2008.14
10. anvisa. Anvisa esclarece sobre indicações do PMMA – cosmetovigilancia – Anvisa. 25/07/2018. 2018;d:12-14. http://antigo.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=4689506&_101_type=content&_101_groupId=219201&_101_urlTitle=anvisa-esclarece-sobre-indicacoes-do-pmma&redirect=http%3A%2F%2Fantigo.anvisa.gov.br%2Fresultado-de-busca%3Fp_p_id%3D3%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Dcolumn-1%26p_p_col_count%3D1%26_3_groupId%3D0%26_3_keywords%3DPolimetilmetacrilato%2B%26_3_cur%3D1%26_3_struts_action%3D%252Fsearch%252Fsearch%26_3_format%3D%26_3_formDate%3D1441824476958&inheritRedirect=true.
11. Chacur R, Sampaio Menezes H, Maria Bordin da Silva Chacur N, et al. Gluteal augmentation with polymethyl methacrylate: A 10-year cohort study. Plast Reconstr Surg – Glob Open. 2019;7(5):1-9. doi:10.1097/GOX.0000000000002193
12. Casavantes L, Lemperle G, Morales P. Penile Girth Enhancement With Polymethylmethacrylate-Based Soft Tissue Fillers. J Sex Med. 2016;13(9):1414-1422. doi:10.1016/j.jsxm.2016.06.008
13. Alter GJ. Editorial Comment on “Penile Girth Enhancement With PMMA-Based Soft Tissue Fillers.” J Sex Med. 2016;13(9):1423. doi:10.1016/j.jsxm.2016.07.011
14. Vardi Y, Harshai Y, Gil T, Gruenwald I. A Critical Analysis of Penile Enhancement Procedures for Patients with Normal Penile Size: Surgical Techniques, Success, and Complications. Eur Urol. 2008;54(5):1042-1050. doi:10.1016/j.eururo.2008.07.080
15. Bizic MR, Djordjevic ML. Penile Enhancement Surgery: An Overview. EMJ Urol. 2016;2010(Band 8):94-100. doi:10.33590/emjurol/10312850
16. Yang DY, Lee WK, Kim SC. Tolerability and efficacy of newly developed penile injection of cross-linked dextran and polymethylmethacrylate mixture on penile enhancement: 6 months follow-up. Int J Impot Res. 2013;25(3):99-103. doi:10.1038/ijir.2012.41
Acknowledgement
The authors would like to extend their heartfelt appreciation to all the individuals who participated in this study. Their voluntary involvement and cooperation were instrumental in the successful execution of this research. We also wish to acknowledge the valuable assistance provided by the staff and collaborators who contributed to various aspects of this project.We thank the statistician Dr. Leonardo Saldanha for the statistical calculationsand the secretary Rosângela Santos for the attention and care given to all the doctors on the team.
Funding Information
This study was conducted without external financial support. All expenses associated with the research, including materials, equipment, and personnel, were borne by the authors.
Disclosure
The authors affirm that there are no conflicts of interest to disclose regarding the publication of this article. The research was conducted impartially, and the findings presented are based solely on the data collected and analyzed during the study period.
Conflict of Interest: The authors declare that there are no conflicts of interest related to the research, authorship, or publication of this article.All authors have read and approved the final version of the manuscript.
