REGISTRO DOI: 10.69849/revistaft/ma10202507311035
Rayssa Martins Souza Hass1
Milka Bringel Batista Farias1
Thiago Naranjo Batista1
Barbara Seabra Carneiro2
SUMMARY
Paracoccidioidomycosis (PCM) is an endemic fungal disease with a geographic distribution limited to Central and South America, more prevalent among men with an average ratio of 13:1. Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by severe immune dysregulation. Alterations in several signal transduction pathways culminate in dysfunction of the immune system as a whole. These alterations are responsible for organ damage, and immunosuppression is the essential therapeutic approach. This approach makes patients susceptible to infections and may clinically mimic other autoimmune pathologies. This case report describes a patient diagnosed with SLE and refractory renal manifestations who developed a clinical picture suggestive of associated ANCA vasculitis. However, she had developed numerous infections during attempts at immunosuppression to control disease activity, the last of which was diagnosed as paracoccidioidomycosis.
Keywords: Paracoccidioidomycosis, infection, systemic lupus erythematosus, immunosuppression and vasculitis.
INTRODUCTION
Paracoccidioidomycosis is an endemic fungal disease with a geographic distribution limited to Central and South America. In Brazil, it is predominantly found in the Southeast, Central-West, and South regions; however, in the 1990s, it spread to the North. Its chronic form is more prevalent among men in their third to sixth decades of life, with an average ratio of 13:1, as estradiol appears to protect adult women from developing the disease, but not from infection.
SLE is an extremely complex autoimmune disease whose pathophysiological basis is severe immune dysregulation. Alterations in various signal transduction pathways and interferon-related pathophysiological signatures result in immune dysfunction. The exacerbated systemic inflammatory process and systemic immune complex deposition are among the causes of functional and structural organ impairment. Immunosuppression aimed at inducing remission is the cornerstone of treatment.
Immunosuppression in patients with autoimmune diseases makes them susceptible to a range of infections. In this population, these pathologies tend to be more severe, have atypical progressions, and can be highly mimicking of other autoimmune etiologies.
This is a case report of a young patient with overlap syndrome (SLE) and antiphospholipid antibody syndrome (APS) who developed a maxillary sinus polyp associated with mucosal thickening and recurrent epistaxis. Given the patient’s existing autoimmune conditions, a third etiology was considered, with ANCA-associated vasculitis being the most likely based on the clinical course. However, the differential diagnosis of infection in these patients should always be ruled out, especially when the condition is atypical, does not improve, or even worsens during immunosuppression.
CASE REPORT
Patient DHSO, a 37-year-old woman, was diagnosed with SLE and APS in 2007. Throughout 2023, she developed foamy urine associated with uncontrolled blood pressure and lower limb edema. Laboratory tests revealed several abnormalities, including positive anti-double-stranded DNA antibody, supplement consumption, urinary routine with cylindruria, leukocyturia, and hematuria, as well as a considerable increase in daily proteinuria. The patient was then diagnosed with diffuse proliferative lupus nephritis. The patient developed rapid loss of renal function resulting in stage IIIB chronic kidney injury even after remission induction therapy with cyclophosphamide, mycophenolate mofetil, tacrolimus, and full anticoagulation with Marevan. A renal biopsy was not performed due to reduced renal volume and a significant bleeding risk, which increased the risk of procedure-related complications. At the same time, the patient developed recurrent epistaxis, sometimes massive, requiring multiple blood transfusions. The etiological investigation of the nasal condition revealed a left maxillary polyp with bilateral maxillary mucosal thickening. Therefore, she was treated as having bacterial rhinosinusitis with several antibiotic regimens, without clinical improvement.
After approximately 10 months, the patient developed an ulcerated lesion on the palate, rapidly progressing to perforation with an oronasal communication. At this point, she was referred to the Otorhinolaryngology department for a nasal biopsy and requested investigation for ANCA-associated vasculitis. It is worth noting that the patient had previously developed several infectious complications, such as cytomegalovirus and toxoplasmosis. Pathology revealed the presence of the fungus Paracoccidioides brasiliensis , and treatment with itraconazole was promptly initiated. Vasculitis was ruled out after extensive investigation. After antifungal treatment, the patient showed satisfactory clinical improvement.
DISCUSSION
PCM is a chronic systemic infection caused by thermodimorphic fungi, with great potential for dissemination in immunocompromised individuals [11,12,14]. In its mucocutaneous form, it can cause extensive tissue destruction, with mucosal ulceration and perforation of anatomical structures, mimicking systemic inflammatory diseases, such as small vessel vasculitis [15,16].
The reported case illustrates the clinical challenge regularly faced by rheumatologists: differentiating between infection and autoimmunity. The distinction between granulomatosis with polyangiitis (GPA) and disseminated PCM is emphasized here. The nasal destruction, massive epistaxis, and oronasal perforation initially suggested a diagnosis of ANCA-associated vasculitis. However, histopathological confirmation of PCM completely altered the therapeutic approach, avoiding intensification of immunosuppression, which could have been even more detrimental.
This type of mimicry between PCM and GPA is described in the literature [17–19]. Table 1 summarizes the main clinical and laboratory distinctions between the two conditions:
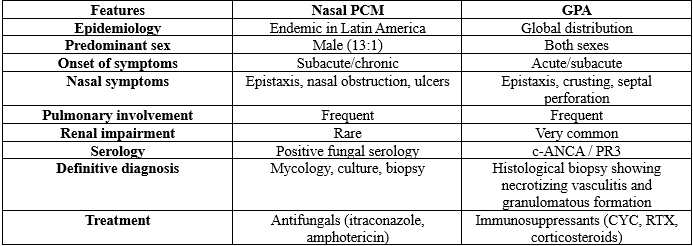
In addition to the clinical presentation, histopathology provides essential findings for differential diagnosis.
Table 2 – Differential histopathological findings:

In endemic regions, even in less prevalent subregions, infections such as PCM should be among the main differential diagnoses of destructive mucocutaneous manifestations in patients with autoimmune diseases [12,15]. Additionally, immunosuppressed patients may present infectious conditions with atypical and disseminated evolution, reinforcing the need for histological confirmation before intensifying immunosuppression [16,17].
ANCA positivity is not exclusive to vasculitis. Several studies indicate that chronic infections, including PCM and tuberculosis, can present with serological positivity, which can further confuse the picture [8,10].
Early recognition of the infection and optimal treatment were crucial to the patient’s good clinical outcome. This case reinforces the importance of ongoing infectious surveillance in the management of autoimmune patients, especially in tropical regions.
CONCLUSION
Patients with immune-mediated diseases and those undergoing immunosuppression are known to be more predisposed to infections. Therefore, these etiologies should often be considered as part of the differential diagnosis, especially in cases of atypical progression. Endemic etiological agents should be primarily ruled out, but epidemiologically less prevalent ones should also receive due consideration before the most likely diagnosis is established. Rheumatologists’ knowledge of this topic is crucial for early diagnosis and treatment, consequently reducing morbidity and unfavorable patient outcomes.
BIBLIOGRAPHICAL REFERENCES
- Restrepo A, Tobón AM, Trujillo J, Restrepo MA, Gómez BL. Paracoccidioidomycosis in Colombia: epidemiological and clinical aspects. Mycopathology. 2021;186(2):165–75. doi:10.1007/s11046-020-00515-0
- Benard G, Duarte AJ, Silva ME. Immunopathology of human paracoccidioidomycosis. Mycopathology. 2020;185(5):931–48. doi:10.1007/s11046-020-00461-x
- Pan American Health Organization (PAHO). Epidemiological update: Paracoccidioidomycosis in the Americas. Washington, DC: PAHO; 2022. [Available at: https://www.paho.org]
- Duarte MI, Pagliari C, Sotto MN. Fungal infections in immunocompromised hosts: histopathology and immunohistochemistry. J Bras Patol Med Lab. 2022;58:e202210. doi:10.5935/1676-2444.202210
- Borba EF, Bonfá E. Immunosuppressive therapy and risk of infection in systemic lupus erythematosus. Lupus. 2020;29(5):559–67. doi:10.1177/0961203320908937
- Mota LMH, Oliveira AC, Lima RA, et al. Recommendations for the management of systemic lupus erythematosus in Brazil: consensus of the Brazilian Society of Rheumatology 2021. Adv Rheumatol. 2021;61:43. doi:10.1186/s42358-021-00201-z
- Cano LE, González Á. Fungal infections and immunosuppressive therapy: focus on paracoccidioidomycosis. J Fungi (Basel). 2021;7(4):253. doi:10.3390/jof7040253
- Sousa Mda G, Martins LC, Araújo SA, et al. Disseminated paracoccidioidomycosis in a patient with systemic lupus erythematosus: case report and review. Rev Soc Bras Med Trop. 2019;52:e20180189. doi:10.1590/0037-8682-0189-2018
- Neves RFS, Silva AB, Barreto GdS, et al. Paracoccidioidomycosis in immunocompromised hosts: a diagnostic and therapeutic challenge. Med Mycol Case Rep 2023;41:47–51. doi:10.1016/j.mmcr.2023.03.003
- Borges AS, Silva GM, Ferreira MS. Paracoccidioidomycosis in immunocompromised patients. Curr Fungal Infect Rep 2022;16:80–9. doi:10.1007/s12281-022-00416-6
- Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017;17(11):e367–e377.
- Shikanai-Yasuda MA, Mendes RP, Colombo AL, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop. 2017;50(5):715–740.
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011;365(22):2110–2121.
- Ferreira MS, Borges AS. Paracoccidioidomycosis. Semin Respir Crit Care Med. 2008;29(2):182–197.
- Silva-Vergara ML, Martinez R. Paracoccidioidomycosis in immunocompromised patients: a review. Mycopathology. 2012;173(2-3):95–102.
- Carvalho MA, et al. Paracoccidioidomycosis as differential diagnosis of destructive upper airway lesions. Braz J Infect Dis. 2003;7(4):263–267.
- Nogueira SA, Oliveira MML, Lima DMM, et al. Clinical and laboratory aspects of patients with paracoccidioidomycosis mimicking Wegener’s granulomatosis. Rev Soc Bras Med Trop. 2011;44(6):758–760.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11.
- Prattes J, Flick H, Prüller F, et al. Paracoccidioidomycosis mimicking systemic vasculitis: a case report and literature review. Mycoses. 2018;61(4):257–260.
- Iudici M, et al. ANCA positivity in infectious diseases. Clin Exp Rheumatol. 2015;33(1 Suppl 89):S108–S110.
1Resident at Getúlio Vargas University Hospital – UFAM
2Preceptor of the Getúlio Vargas University Hospital – UFAM
