REGISTRO DOI: 10.5281/zenodo.7999850
Luciane Neris Cazella1
Anderson Razzé Marinho2
Adriane Viapiana Bossa3
Angela Mara Rambo Martini3
Gustavo David dos Santos4
ABSTRACT
Introduction: Urinary tract infections (UTIs) are amongst the most common pathological conditions in community and hospital environments. The bacterium Escherichia coli is its main etiological agent. The conventional medical treatment includes antibiotics; however, it is necessary to search for strategies that can avoid microbial resistance. This highlights the importance of Medicinal Biomagnetism (MB), a therapeutic system developed in 1988 by the Mexican physician Dr. Isaac Goiz Durán (1941-2021), which consists of the use of magnets in pairs, with magnetic flux greater than 1,000 Gauss, in topographic points that present biomagnetic resonance and aims to correct distortions in the body’s pH, helping to restore and maintain the Normal Energy Level (NEL). Objective: To evaluate people with UTIs caused by E. coli who were treated with MB. Methodology: MB treatment was carried out in two female participants (47 and 50 years old) and one male participant (54 years old), who had positive urine culture results for E. coli. The participant also had a high dose of total prostate specific antigen (PSA). Only the first case required a second MB session. Laboratory tests were repeated. Results and discussion: The urine culture tests indicated that there was no bacterial growth in cases treated with MB. And, the dosage of total PSA, indicated a reduction from 6.13 to 0.54 ng/mL. This study demonstrated that people showed elimination of UTIs caused by E. coli when treated with MB. It is suggested that this technique be researched in larger trials to better determine its contribution to the treatment of UTIs.
Keywords: Medicinal Biomagnetism, Biomagnetic Pair, Uropathogenic Escherichia coli, urinary tract infections, non-antibiotic drugs.
1 – INTRODUCTION
Urinary tract infections (UTIs) are amongst the most common pathological conditions (ZALEWSKA-PIĄTEK; PIĄTEK, 2020) and can affect patients’ quality of life (CAI; KONSTANTINIDIS; WARD, 2021). UTIs are caused by a variety of pathogens, with emphasis on Escherichia coli, Enterococcus spp., Klebsiella pneumoniae, Candida spp. and Proteus mirabilis (MEDINA; CASTILLO-PINO, 2019). About 80% of community-transmitted UTIs and 40-50% of hospital-transmitted UTIs are caused by uropathogenic E. coli strains (SUDARSHAN et al., 2021). The prevalence of E. coli (58.9%) was found by Masson et al. (2020), with predominant infection in people between 13 and 59 years old (63.3%), mainly in females (85.8%).
Such infections frequently occur in women due to anatomical differences, physiological changes in pregnancy and menopause (KRISHNASWAMY; BASU, 2020), as well as, in patients undergoing urinary tract instrumentation (MITCHELL et al., 2021), cases of diabetes mellitus (KAMEI; YAMAMOTO, 2021), hypoestrogenism (CHEN; SU; LAU, 2020), immunosuppression (TANDOGDU et al., 2016), autoimmune diseases (MATAS et al., 2020) and urinary incontinence (MELO et al., 2017). The clinical manifestation of the infection may present as pyelonephritis, cystitis, urethritis, and prostatitis, with signs and symptoms that may vary, such as dysuria, polyuria, overactive bladder, fever, chills, suprapubic or lumbar pain (BRASIL, 2013). Severe sequelae include recurrent infection, pyelonephritis with sepsis, premature delivery in pregnant women, and complications from frequent use of antimicrobials (FLORES-MIRELES et al., 2015).
UTIs are clinically treated with antibiotics, however, there is an increase in resistance to these drugs among urinary tract pathogens (ROSSI et al., 2020; ZHU et al., 2021), which requires the search for non-antimicrobial options for the control and prevention (FLORES-MIRELES et al., 2015; RODRIGUEZ-MAÑAS, 2020). This resistance causes repetition and chronicity of the infection, with the appearance of more serious diseases (TERLIZZI; GRIBAUDO; MAFFEI, 2017). The National Health Surveillance Agency in Brazil (BRASIL, 2019) reports microbial resistance of E. coli, in UTI treatments, in 24.41 and 24.54% in adult and pediatric Intensive Care Units, respectively; mainly to 3rd and/or 4th generation Cephalosporin antimicrobials and Carbapenems.
According to González-Villalobos et al. (2021), the pathogenesis of E. coli includes the stages of bacterial adherence to the urogenital epithelium and biofilm production. In the first phase, it binds to glycosylated surface proteins using type 1 fimbriae. This binding leads to invasion of bladder epithelial cells by E. coli, which can escape into the cytoplasm and form intracellular bacterial communities. In the second phase, biofilm formation allows bacteria to have high resistance to antibiotics and normal urine flow.
It is estimated that more than 150 million people develop UTIs each year worldwide (SCAGLIONE; MUSAZZI; MINGHETTI, 2021). Although some patients are diagnosed and treated efficiently, many may not seek diagnostic evaluation or treatment. In other cases, microbial resistance may occur (QINDEEL et al., 2021), as well as recurrence related to the virulence of pathogens and patient condition (CAI, 2021). The rapid dissemination of multiresistant microorganisms requires effective preventive approaches (GUCLU et al., 2021).
In view of this, the importance of Medicinal Biomagnetism (MB) is highlighted due to its contribution to the treatment of pathologies, including infectious diseases (HÍLU; GOIZ DURÁN; MENDOZA CASTELÁN, 2009; GOIZ DURÁN, 2016; FRANK, 2017; DAMYANOV et al., 2019a, b). MB is a therapeutic system developed by the Mexican physician Dr. Isaac Goiz Durán (1941-2021) from 1988, which involves the identification of energy imbalances in the body and the positioning of pairs of magnets, in specific topographic points that are in biomagnetic resonance (GOIZ MARTÍNEZ, 2018). The objective of the technique is to promote homeostasis in the body; prevent and treat pathological conditions, facilitate the restoration and maintenance of health condition, providing the body with balanced bioelectricity (CASTEJÓN, 2012b; GOIZ MARTÍNEZ, 2017a). MB can be integrated into the medical treatment of diseases to reduce symptoms and rehabilitate body functions (GOIZ MARTÍNEZ, 2017b).
According to Goiz Durán (2008), MB studies the bioenergetic phenomena produced by microorganisms and glandular dysfunctions in the body, because for their implantation, metabolism and reproduction, distortions of the hydrogen potential (pH) of the organs that support them must occur. The restoration and maintenance of a state of bioelectric balance in the body is related to the principles of magnetism, pH concepts, biomagnetic resonance, entropy, symbiosis, universal law of charges and homeostasis. Pathological and pathogenic manifestations are formed from the polarization of biochemical substances resonant with each other when they exceed the limits of organic entropy, that is, a Biomagnetic Pair (BMP) that makes it possible to identify each pathology. When an organ goes out of its normal energy level, medium-intensity magnetic fields can measure these distortions, causing shortening or lengthening of the right hemibody. Shortening of the right hemibody occurs due to the interaction of a magnetic field of defined polarity with the biomagnetic charge of an organ when it is distorted to an abnormal alkaline pH, and the right hemibody lengthens in the presence of an organ with acidic pH. The left hemibody does not undergo these variations because it is traversed 80 times per minute, under normal conditions, by an electromagnetic current generated by the activity of the heart.
The south biomagnetic pole is formed by the excess of hydrogen ions, where the pH becomes more acidic, favoring the presence of viruses; and the north, due to the deficit of hydrogen ions and the presence of hydroxyls, where the pH becomes more alkaline, favoring the presence of pathogenic bacteria (GOIZ DURÁN; MENDOZA CASTELÁN; MENDOZA CASTELÁN, 2005).
According to Goiz Durán (2008), BMP is the set of charges that identify a pathology, and which is made up of two main charges of opposite polarity, which are formed at the expense of the fundamental alteration of the pH of the organs that sustain it. From this bioenergetic duality another fundamental principle arises, called Normal Energy Level (NEL). The NEL defines the bioenergetic limits where the cellular metabolic processes of human organisms occur and which, due to the temperature, cannot exceed the limit of just one degree centigrade (36 to 37ºC). The BMP is composed of NEL and the two biomagnetic poles in vibrational and energetic resonance, reciprocal in terms of the concept of energy. Regarding the pH, it is close to the neutral value of the conventional scale, with a tolerance of three tenths in both directions. The identification of the biomagnetic poles follows the energetic resonance of the magnetic pole with that of the organism studied. Within the limit of the NEL there is no measurable manifestation.
According to Goiz Martínez (2017b), BMPs can be classified into regular and special. Regular BMPs are related to specific pathogenic microorganisms (viruses, bacteria, fungi, or parasites). Special BMPs are not directly related to pathogenic microorganisms and are divided into: dysfunctional (glandular dysfunctions, dysfunctions of tissues, organs and systems of the body), psycho-emotional (problems of the psyche sphere), complex (chronic-degenerative, autoimmune, metabolic diseases, tumors, poisoning, injuries and sequelae of the disease; they can be used as a complement in the treatment of various diseases); and, reservoirs (may temporarily harbor pathogenic microorganisms related to regular BMPs).
Evidence indicates the seriousness with which UTIs must be tackled due to their possible complications. And, their treatment remains challenging due to resistance to conventional antimicrobial therapy, showing the importance of new strategies to address the problem. Thus, the aim of this study was to evaluate people with UTIs caused by E. coli who were treated with MB.
2 – METHODOLOGY
The research was conducted in a MB clinic, in Brusque, Santa Catarina, with two female participants (47 and 50 years old) and one male participant (54 years old), symptomatic, with clinical evaluation of UTI caused by E. coli. They had no other associated diseases and did not use medication.
The three participants underwent urine culture tests in certified laboratories, before and after treatment. The male participant also performed the total prostate-specific antigen (PSA) test.
MB treatment was applied, as described by Goiz Durán (2008) and Goiz Martínez (2018). This methodology (Figure 1) involves three main procedures: complete scanning, impaction, and depolarization. Complete scanning consists of reviewing, through a kinesiological test, topographic points on the body, with the aid of a scanning worksheet (GOIZ DURÁN, 2008; GOIZ MARTÍNEZ, 2017 a, b; BOSSA, 2021a). Impaction consists of positioning pairs of magnets in the resonances identified in the scanning and the depolarization process is completed in approximately 15-30 minutes.
Figure 1 – Methodology of the Medicinal Biomagnetism technique.
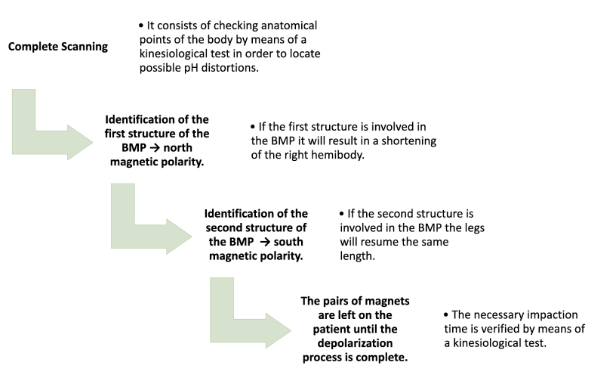
The best way to track the biomagnetic poles is with the patient in supine position, on a firm base, made of wood or insulating material to avoid interference with the magnets. It is not necessary to take off the patient’s shoes, because the heel provides the reference for assessing the shortening or lengthening of the right lower limb. But it can be done without shoes, placing a mark on the heel as a reference for measurement.
The north magnetic polarity identifies the first structure of a BMP. If it is involved, there is a shortening of the right hemibody, called the Magnet-foot reflex, when placing the black/negative magnet (technical convention for the north pole) over the structure. In sequence, the second structure of the BMP is identified with the south magnetic polarity. When placing the red/positive magnet (technical convention for the south pole), if the second structure is involved in the BMP, the legs resume the same length.
The left hemibody remains fixed in its length, while the right presents the manifestations of stretching or contraction with which the altered organ can be defined as well as its polarity. One or more BMPs related to the patient’s symptoms and signs, or other conditions identified in the scanning can be treated. Once the BMPs involved are identified, the magnets are placed on the patient, for as long as their energy tests indicate to be necessary, until the depolarization process is completed. The kinesiological test is used to verify that the identified BMP has been depolarized. The time of BMP depolarization varies due to factors such as age, patient’s energy status and geographic location. Depolarization is verified by removing the positive magnet and revising the symmetry. Depolarization occurs by applying a magnetic field of opposite polarity to the bioenergetic field produced by organisms to attract hydrogen or hydroxyl ions of opposite polarity to the outside of the body.
Case 1: In October 2020, a female sought care of MB, 47 years old, married, in menopause, with proven hormonal alteration and medical history of UTI. She claimed that the UTI episodes started in 2005, six months after the second delivery, with symptoms of high fever. She began to have two to three infections per year, in which the E. coli bacteria was detected in a urine culture with antibiogram. Since March 2019, she has had monthly exams with positive results for the E. coli bacteria. Ultrasound exams and renal scintigraphy showed mild dysfunction in the right kidney. She reported previous use of antibiotics tested by antibiogram and of immunotherapy, prebiotics and probiotics indicated for the prevention of recurrent UTIs, however, the laboratory diagnosis was still positive. She had not used medication for two months. The participant underwent a urine culture test, which indicated E. coli bacteria, with a colony count greater than 100,000 CFU/mL, on October 15, 2020. In the first session for treatment with MB, the BMP Thymus-Rectum and Indicator-Indicator identified in full screening were impacted for 30 minutes. As a new urine culture test, on November 19, 2020, showed a positive result for E. coli, a second MB session was performed. This time, the BMP Thymus-Rectum and, in addition to Index-Indicator, the BMP of the other fingers of the hands – Thumb-Thumb, Middle Finger-Middle Finger, Ring Finger-Ring finger, Little Finger-Little Finger – were impacted. A new urine culture exam was performed on January 7, 2021.
Case 2: In February 2021, a female sought treatment of MB, 50 years old, married, with symptoms of pollakiuria, abdominal pain, dysuria, and with positive urine culture results for the E. coli bacteria for two months. The participant underwent a urine culture test, which indicated E. coli bacteria, with a colony count greater than 100,000 CFU/mL, on February 17. The BMPs Thymus-Rectum and Indicator-Indicator identified in the complete scanning were impacted for 30 minutes. On March 11, a new urine culture was performed.
Case 3: In March 2021, a 54-year-old man, married, presented with symptoms of pollakiuria, overactive bladder, suprapubic pain, dysuria, nocturia, change in urine color and appearance. He had previous histories of total PSA dosages of 3.77 and 8.84 ng/mL in June and August 2020, respectively. On March 8, 2021, the urine culture revealed the presence of E. coli, with a colony count greater than 100,000 CFU/mL, and the total PSA dosage indicated 6.13 ng/mL. Complete scanning was performed and the BMPs Thymus-Rectum and Indicator-Indicator were impacted for 30 minutes. The participant repeated the urine culture exams on March 23 and the total PSA dosage on April 9.
The Informed and Free Decision Consent (IFDC) for Case Reports was signed by the participants to provide data collection used in this study.
3 – RESULTS AND DISCUSSION
The BMPs identified during the complete scanning of the participants were the Indicator-Indicator, which has been found to be the preferred pair of E. coli bacteria (GOIZ DURÁN; MARTÍNEZ; MENDOZA CASTELÁN, 2010; CASTEJÓN, 2012a; GOIZ MARTÍNEZ , 2017a; DE MONDELO, 2017; GOIZ MARTÍNEZ, 2019) and Thymus-Rectum, associated with strengthening the immune system and E. coli bacteria (GOIZ DURÁN, 2007; CASTEJÓN, 2012a; GOIZ MARTÍNEZ, 2017a). When no result is obtained with the Indicator-Indicator BMP, it is recommended to check whether there is a need to impact the BMPs of the other fingers (BOSSA, 2021a).
To this date, several BMPs have been identified as associated with the urinary system, as well as BMPs required for strengthening the immune system. These pairs include Kidney-Kidney, Bladder-Bladder, Thymus-Rectum, Supraspinatus-Supraspinatus, Inguinal Nerve-Inguinal Nerve, Minor Trochanter-Minor Trochanter, Adductor-Adductor, Ureter-Ureter, Descending Colon-Descending Colon, Coccyx-Coccyx, Urethra-Urethra, Testicle-Testicle, Vagina-Vagina, Vas Deferens-Larynx, Spermatic Conduit-Spermatic Conduit, Thymus-Thymus, Appendix-Thymus, Suprarenal-Adrenal, among others (GOIZ DURÁN, 2008; GOIZ MARTÍNEZ, 2017a; GOIZ MARTÍNEZ, 2019).
Goiz Martínez (2017b) defines BMP as the set of dysfunctional bioelectric charges related to a pathology, composed of two main charges of opposite polarity, which are formed at the expense of the bioelectric alteration and pH of the tissues or organs that sustain them in an organism. Thus, the charges are bioelectric because they are found in the organism; and, dysfunctional, since they are capable of producing a phenomenon of pelvic limb dysmetria (PLD), if a magnet is specifically positioned on them. These charges, when removed (depolarized), no longer induce the PLD phenomenon, and the magnets are capable of producing a therapeutic effect.
In case 1, the participant had well-established risk factors for UTI, including female sex, menopause, hormonal changes and, most notably, a history of UTIs (RODRIGUEZ-MAÑAS, 2020). Even with all these factors, in just two MB sessions, the urine culture result indicated that there was no bacterial growth. Physiological changes in premenopausal or postmenopausal women are associated with an increased risk of developing UTIs (ROSSI et al., 2020). The decrease in estrogen levels reduces the ability to resist bacterial colonization, alters the vaginal flora and increases the vaginal pH (PEREZ-CARRASCO et al., 2021). UTIs are classified as recurrent when three or more episodes occur in 12 months (VENKATESAN et al., 2020). The decrease in estrogen is associated with conditions that can promote recurrent UTIs in postmenopausal women, such as urinary incontinence, cystocele, and post-vesical residue (RODRIGUEZ-MAÑAS, 2020). The high rates of recurrent UTIs suggest that antibiotics may not be effective for all cases (FLORES-MIRELES et al., 2015), which requires rapid diagnosis, treatment and prevention strategies that avoid antibiotic resistance (PETCA et al., 2020). Exams performed by the participant indicated a slight dysfunction in the right kidney. UTIs can lead to more serious consequences, in particular kidney damage and kidney failure (KANA et al., 2020).
In case 2, after the impaction of the Thymus-Rectum and Indicator-Indicator PBM, the urine culture examination indicated that there was no bacterial growth. The case included the female gender factor; another factor may be related to the innate immune response, the main defense mechanism against infectious agents in the urinary tract (LACERDA MARIANO; INGERSOLL, 2020).
For case 3, there was also a need to impact the BMP Thymus-Rectum and Indicator-Indicator. After this treatment, the result of the urine culture test indicated that there was no bacterial growth. And the total PSA dosage showed a reduction from 6.13 to 0.54 ng/mL. Generally, a threshold of 4 ng/mL is used as a reference for PSA (INSTITUTO NACIONAL DE CÂNCER JOSÉ ALENCAR GOMES DA SILVA, 2021). Case 3 had risk factors related to previous histories of total PSA measurements. The risk of UTI for men increases after the age of 50 when they are more likely to develop prostate problems due to loss of prostatic fluid (JOHN; MBOTO; AGBO, 2016), or associated with prostate diseases (BRASIL, 2013; LACERDA MARIANO; INGERSOLL, 2020). These reduce the antimicrobial properties of the prostatic liquid and obstruct the urinary flow (COSTA et al., 2019). Benign prostatic hyperplasia is one of the main causes of lower urinary tract obstruction, and this bladder outlet obstruction can lead to UTIs.
The MB proposes a comprehensive review of the organism (biomagnetic scanning) and the application of the magnet poles occurs on the same poles as the BMP, that is, the site of pH distortions (PERSON, 2016). The BMP makes it possible to identify the organ that is generating it, the polarity, the related virus and bacteria, as well as the interaction of two or more of these microorganisms, making the therapeutic procedure precise (BAILEY, 2010). The pathology is identified in its etiology, pH changes are simultaneously corrected, and cellular and organic health is conditioned (GOIZ DURÁN, 2014). In the treatment with MB, it is recommended to carry out scannings of pets (BOSSA, 2021b), as there is evidence that cats and dogs have the potential for infection and dissemination of multidrug-resistant E. coli related to those that cause UTI in humans (ZOGG et al., 2018; BRILLIANTE et al., 2020).
For the three cases studied, treatment with MB enabled the elimination of UTIs caused by E. coli. Consequently, it promoted the recovery of the quality of life, impaired by the symptoms. In this article, a brief approach to MB was used in order to assess its contribution to the treatment of UTIs. Possibly, comprehensive examinations and a greater number of sessions, with the impact of other potentially active BMPs, result in more clinical benefits. In addition to the rapid effectiveness of the protocol, the low cost of the treatment ensures its accessibility, indicating that it could widely help the population.
4 – CONCLUSION
From the results obtained, it was verified that the BMPs Indicator-Indicator and the ones formed with the other fingers of the hands – Thumb-Thumb, Middle Finger-Middle Finger, Ring Finger-Ring finger, Little Finger-Little Finger, correspond to the enterobacter E. coli. as well as BMP Thymus-Rectum, which is related to strengthening the immune system and E. coli. This study demonstrated that the participants had the elimination of UTIs, caused by E. coli, when treated with MB. Thus, large-scale research on the use of MB, with clinical and laboratory tests, to reduce/eliminate UTIs are important to ratify the importance of the technique.
REFERENCES
BAILEY, Janice. Bioenergetic Basics: The art of dynamic wellness with Goiz Biomagnetic Pairs. Charleston, SC: BookSurge Publishers; 254p., 2010.
BOSSA, Adriane Viapiana. Apostila: Curso de Biomagnetismo Medicinal. 12. ed. Cascavel: Instituto Par Magnético, 114 p., 2021a.
BOSSA, Adriane Viapiana. Biomagnetismo Medicinal Avançado, Bioenergética e Desbloqueio Emocional Magnético Avançados. 2. ed. Cascavel: Instituto Par Magnético, 281 p., 2021b.
BRASIL. Agência Nacional de Vigilância Sanitária – Anvisa. Boletim segurança do paciente e qualidade em serviços de saúde nº 22: avaliação dos indicadores nacionais de Infecções Relacionadas à Assistência à Saúde (IRAS) e Resistência Microbiana (RM), Ano 2019. Brasília: Anvisa, 2019.
BRASIL. Agência Nacional de Vigilância Sanitária – Anvisa. Microbiologia clínica para o controle de infecção relacionada à assistência à saúde. Módulo 3: Principais Síndromes Infecciosas/Agência Nacional de Vigilância Sanitária. Brasília: Anvisa, 150 p., 2013.
BRILHANTE, Michael; MENEZES, Juliana; BELAS, Adriana; FEUDI, Claudia; SCHWARZ, Stefan; POMBA, Constança; PERRETEN, Vincent. OXA-181-producing extra-intestinal pathogenic Escherichia coli ST410 isolated from a dog in Portugal. Antimicrobial Agents and Chemotherapy, v. 64, n. 4, p. 1-14, 2020.
CAI, Tommaso; KONSTANTINIDIS, Cristian; WARD, Sam. A non-pharmacological approach to the treatment of urinary tract infections: case reports with Utipro® Plus. Drugs in Context, v. 10, p. 1-7, 2021.
CAI, Tommaso. Recurrent uncomplicated urinary tract infections: definitions and risk factors. GMS Infectious Diseases, v. 9, 2021.
CASTEJÓN, Enrique de Juan Gonzalez. Microbiología y Par Biomagnético. 1. ed. Madrid: Colección Anuna, Espanha, 66 p., 2012a.
CASTEJÓN, Enrique de Juan Gonzalez. Par Biomagnético y la Medicina Germánica. 1. ed. Madrid: Colección Anuna, Espanha, 36 p., 2012b.
CHEN, Ying-Yu; SU Tsung-Hsien; LAU Hui-Hsuan. Estrogen for the prevention of recurrent urinary tract infections in postmenopausal women: a meta-analysis of randomized controlled trials. International Urogynecology Journal, v. 32, n. 1, p. 17-25, 2020.
CHOI, Jin Bong; MIN, Seung Ki. Complicated urinary tract infection in patients with benign prostatic hyperplasia. Journal of Infection and Chemotherapy, S1341-321X, n. 21, 00167-7, 2021.
COSTA, Igor Augusto Costa; DA MATA, Mabeli Ribeiro; DE SOUZA, Mayra Costa; PINTO, Samara Custódio Vieira; MAGALHÃES, Shamara Wayne Ferreira; MENDES, Thamires Garcia Rocha; COSTA, Viviane Torres; DA MOTTA, Patrícia Gonçalves; DE OLIVEIRA, Maria Emília. Infecção do trato urinário causada por Escherichia coli: revisão de literatura. Salusvita, Bauru, v. 38, n. 1, p. 155-193, 2019.
DAMYANOV, Christo; MASLEV, Ivan; PAVLOV, Vladimir; TODOROV, Alexander. A new treatment method of Advanced Metastatic Tumors. Annals of Clinical Case Reports. v. 4, n. 1647, p. 1-6, 2019a.
DAMYANOV, Christo; MASLEV, Ivan; PAVLOV, Vladimir; TODOROV, Alexander. Integrative oncology at the clinicist’s look chronology for the creation and development of the IPT & BMP Method for treatment of oncological diseases. Clinics in Oncology, v. 4, n. 1671, p. 1-5, 2019b.
DE MONDELO, Salvador Gutiérrez Rodriguez. Atlas del par biomagnético del Dr. Isaac Goiz Durán. Baleares: Salud y Bioestética, 360 p., 2. ed., 2017.
FLORES-MIRELES, Ana L.; WALKER, Jennifer N.; CAPARON, Michael; HULTGREN, Scott J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature Reviews Microbiology, London, v. 13, n. 5, p. 269-284, 2015.
FRANK, Bryan L. Biomagnetic Pair Therapy and typhoid fever: a pilot study. Medical Acupuncture, v. 29, n. 5, p. 308-312, 2017.
GOIZ DURÁN, Isaac. El Par Biomagnético. 5. ed. Chapingo, México D. F.: Universidad Autónoma Chapingo, 171 p., 2008.
GOIZ DURÁN, Isaac. El sida es curable. 3. ed. Chapingo, México D. F.: Universidad Autónoma Chapingo, 129 p., 2007.
GOIZ DURÁN, Isaac. Fisiopatología bioenergética. México City, México: Medicinas Alternativas y Rehabilitación S. A. de CV, 362p., 2014.
GOIZ DURÁN, Isaac; MARTÍNEZ, Xabier Zabala; MENDOZA CASTELÁN, Guillermo. El codigo patogeno. Chapingo, México D. F.: Universidad Autónoma Chapingo, 2010.
GOIZ DURÁN, Isaac; MENDOZA CASTELÁN, Guillermo; MENDOZA CASTELÁN, Pedro. Par Biomagnético, Biomagnetismo Médico y Bioenergética, experiencias de curación, año 2005, tomo I. Chapingo, México D. F.: Universidad Autónoma Chapingo, 2005.
GOIZ DURÁN, Isaac. The tumoral phenomenon. México City, México: Medicinas Alternativas y Rehabilitación S. A. de CV, 200p., 2016.
GOIZ MARTÍNEZ, David. Anatomía del Par Biomagnético. Ciudad de México: Biomagnetism Research Institute, 139 p., 2019.
GOIZ MARTÍNEZ, David. Guía de Pares Biomagnéticos primer nivel. Ciudad de México: Biomagnetism Research Institute, 142 p., 2017a.
GOIZ MARTÍNEZ, David. Guía de Pares Biomagnéticos segundo nivel. Ciudad de México: Biomagnetism Research Institute, 120 p., 2017b.
GOIZ MARTÍNEZ, David. Manual del biomagnetista. Ciudad de México: Biomagnetism Research Institute, 169 p., 2018.
GOIZ MARTÍNEZ, David. Protocolos de Biomagnetismo. Ciudad de México: Biomagnetism Research Institute, 2019.
GONZÁLEZ-VILLALOBOS, Edgar; RIBAS-APARICIO, Rosa María; MONTEALEGRE, Gerardo Erbey Rodea; BELMONT-MONROY, Laura; ORTEGA-GARCÍA, Yerisaidy; APARICIO-OZORES, Gerardo; BALCÁZAR, José Luis; ESLAVA-CAMPOS, Carlos Alberto; HERNÁNDEZ-CHIÑAS, Ulises; MOLINA-LÓPEZ, José. Isolation and characterization of novel bacteriophages as a potential therapeutic option for Escherichia coli urinary tract infections. Applied Microbiology and Biotechnology, v. 105, p. 5617-5629, 2021.
GUCLU, Ertugrul; HALIS, Fikret; KOSE, Elif; OGUTLU, Aziz; KARABAY, Oğuz. Risk factors of multidrug-resistant bacteria in community-acquired urinary tract infections. African Health Sciences, v. 21, n. 1, p. 214-219, 2021.
HILU, Raymond; DURÁN, Isaac Goiz; CASTELÁN; Guillermo Mendoza. Par Biomagnético, Hongos, Virus, Bacterias y Parásitos. 1. ed. Texcoco: Impresiones Emmanuel, 2009.
INSTITUTO NACIONAL DE CÂNCER JOSÉ ALENCAR GOMES DA SILVA. Detecção precoce do câncer / Instituto Nacional de Câncer José Alencar Gomes da Silva. Rio de Janeiro: INCA, 72 p., 2021.
JOHN, Anuli S.; MBOTO, Clement I.; AGBO, Basseye. A review on the prevalence and predisposing factors responsible for urinary tract infection among adults. European Journal of Experimental Biology, v. 6, n. 4, p. 7-11, 2016.
KAMEI, Jun; YAMAMOTO, Shingo. Complicated urinary tract infections with diabetes mellitus. Journal of Infection and Chemotherapy, v. 27, n. 8, p. 1131-1136, 2021.
KANA, Sreerag; GANESH, Rajesh Nachiappa; SURENDRAN, Deepanjali; KULKARNI, Rajendra G.; BOBBILI, Ravi Kishore; JEBY, Jose Olickal. Urine microscopy and neutrophil lymphocyte ratio are early predictors of acute kidney injury in patients with urinary tract infection. Asian Journal of Urology, v. 8, n. 2, p. 220-226, 2020.
KRISHNASWAMY, Priyanka H.; BASU, Maya. Urinary tract infection in gynaecology and obstetrics. Obstetrics, Gynaecology & Reproductive Medicine, v. 30, n. 9, p. 276-282, 2020.
LACERDA MARIANO, Livia; INGERSOLL, Molly A. The immune response to infection in the bladder. Nature Review Urology, v. 17, p. 439-458, 2020.
MASSON, Letícia Carrijo; MARTINS, Luíza Vieira; GOMES, Clayson Moura; CARDOSO, Alessandra Marques. Laboratory diagnosis of urinary tract infections: relation betwenn uroculture and urinalysis. Brazilian Journal of Clinical Analyses, v. 52, n. 4, 2020.
MATAS, Ana; XIPELL, Marc; BODRO, Marta; CERVERA, Ricard; QUINTANA, Luis F. Chapter 3 – Urinary tract infection and autoimmune diseases. In: ATZENI, Fabiola; GALLOWAY, James B.; GOMEZ-REINO, Juan J.; GALLI, Massimo. Handbook of Systemic Autoimmune Diseases. London: Elsevier, v. 16, p. 49-57, 2020.
MEDINA, Martha; CASTILLO-PINO, Edgardo. An introduction to the epidemiology and burden of urinary tract infections. Therapeutic Advances in Urology, v. 11, p. 3-7, 2019.
MELO, Laís Samara de; ERCOLE, Flávia Falci; OLIVEIRA, Danilo Ulisses de; PINTO Tatiana Saraiva; VICTORIANO, Mariana Avendanha; ALCOFORADO, Carla Lúcia Goulart Constant. Urinary tract infection: a cohort of older people with urinary incontinence. Revista Brasileira de Enfermagem, v. 70, n. 4, p. 838-44, 2017.
MITCHELL, Brett; CURRYER, Cassie; HOLLIDAY, Elizabeth; RICKARD, Claire M.; FASUGBA, Oyebola. Effectiveness of meatal cleaning in the prevention of catheter-associated urinary tract infections and bacteriuria: an updated systematic review and meta-analysis. BMJ Open, v. 11, n. 6, p. 1-11, 2021.
ÖZTÜRK, Recep; MURT, Ahmet. Epidemiology of urological infections: a global burden. World Journal of Urology, v. 38, n. 11, p. 2669-2679, 2020.
PEREZ-CARRASCO, Virgínia; SORIANO-LERMA, Ana; SORIANO, Miguel; GUTIÉRREZ-FERNÁNDEZ, José; GARCIA-SALCEDO, José A. Urinary microbiome: Yin and Yang of the urinary tract. Frontiers in Cellular and Infection Microbiology, v. 11, n. 617002, 2021.
PERSON, Ivan. Biomagnetismo e bioenergia magnética: uma nova medicina integrativa. Campinas/SP: Gracioli, 192 p., 2016.
PETCA, Răzvan-Cosmin; MAREȘ, Cristian; PETCA, Aida; NEGOIȚĂ, Silvius; POPESCU, Răzvan-Ionut; BOȚ, Mihaela; BARABÁS, Eniko; CHIBELEAN, Călin Bogdan. Spectrum and antibiotic resistance of uropathogens in Romanian females. Antibiotics, v. 9, n. 8, 472, p. 1-16, 2020.
QINDEEL, Maimoona; BARANI, Mahmood; RAHDAR, Abbas; ARSHAD, Rabia; CUCCHIARINI, Magali. Nanomaterials for the diagnosis and treatment of urinary tract infections. Nanomaterials, v. 11, n. 2, 2021.
RODRIGUEZ-MAÑAS, Leocadio. Urinary tract infections in the elderly: a review of disease characteristics and current treatment options. Drugs in Context, v. 9, n. 2020-4-13, p.1-8, 2020.
ROSSI, Patricia de; CIMERMAN, Sergio; TRUZZI, José Carlos; CUNHA, Clóvis Arns da; MATTAR, Rosiane; MARTINO, Marinês Dalla Valle; HACHUL, Maurício; ANDRIOLO, Adagmar; VASCONCELOS NETO, José Ananias; PEREIRA-CORREIA, João Antônio; MACHADO, Antonia M. O.; GALES, Ana Cristina. Joint report of SBI (Brazilian Society of Infectious Diseases), FEBRASGO (Brazilian Federation of Gynecology and Obstetrics Associations), SBU (Brazilian Society of Urology) and SBPC/ML (Brazilian Society of Clinical Pathology/Laboratory Medicine): recommendations for the clinical management of lower urinary tract infections in pregnant and non-pregnant women. The Brazilian Journal of Infectious Diseases, v. 24, n. 2, p. 110-119, 2020.
SCAGLIONE, Francesco; MUSAZZI, Umberto M.; MINGHETTI, Paola. Considerations on D-mannose mechanism of action and consequent classification of marketed healthcare products. Frontiers in Pharmacology, v. 12, n. 636377, 2021.
SUDARSHAN, Sushmita; HOGINS, Jacob; AMBAGASPITIYE, Sankalya; ZIMMERN, Philippe; REITZER, Larry. The nutrient and energy pathway requirements for surface motility of nonpathogenic and uropathogenic Escherichia coli. Journal of Bacterioly, v. 203, n. 11, 2021.
TANDOGDU, Zafer; CAI Tommaso; KOVES Bela; WAGENLEHNER, Florian; BJERKLUND-JOHANSEN, Truls Erik. Urinary tract infections in immunocompromised patients with diabetes, chronic kidney disease, and kidney transplant. European Urology Focus, v. 2, n. 4, p. 394-399, 2016.
TERLIZZI, Maria E.; GRIBAUDO, Giorgio; MAFFEI, Massimo E. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Frontiers in Microbiology, v. 8, n. 1566, p. 1-23, 2017.
VENKATESAN, Aradhana M.; OTO, Aytekin; ALLEN, Brian C.; AKIN, Oguz; ALEXANDER, Lauren F.; CHONG, Jaron; FROEMMING, Adam T.; FULGHAM, Pat F.; GOLDFARB, Stanley; GETTLE, Lori Mankowski; MARANCHIE, Jodi K.; PATEL, Bhavik N.; SCHIEDA, Nicola; SCHUSTER, David M.; TURKBEY, Ismail B.; LOCKHART, Mark E. ACR Appropriateness Criteria® recurrent lower urinary tract infections in females. Journal of the American College of Radiology, v. 17, n. 11, Supplement, p. S487-S496, 2020.
ZALEWSKA-PIĄTEK, Beata; PIĄTEK, Rafal. Phage Therapy as a novel strategy in the treatment of urinary tract infections caused by E. Coli. Antibiotics (Basel), v. 9, n. 6, 304, p.1-21, 2020.
ZHU, Hongying; CHEN, Yanhui; HANG, Yaping; LUO, Hong; FANG, Xueyao; XIAO, Yanping; CAO, Xingwei; ZOU, Shan; HU, Xiaoyan; HU, Longhua; ZHONG, Qiaoshi. Impact of inappropriate empirical antibiotic treatment on clinical outcomes of urinary tract infections caused by Escherichia coli: a retrospective cohort study. Journal of Global Antimicrobial Resistance, S2213-7165, n. 21, 2021.
ZOGG, Anna Lena; ZURFLUH, Katrin; SCHMITT, Sarah; NÜESCH-INDERBINEN, Magdalena; STEPHAN, Roger. Antimicrobial resistance, multilocus sequence types and virulence profiles of ESBL producing and non-ESBL producing uropathogenic Escherichia coli isolated from cats and dogs in Switzerland. Veterinary Microbiology, v. 216, p. 79-84, 2018.
1Studian Graduate Program in Biomagnetism and Bioenergy Applied to Health, Par Magnético Institute – IPM / University Center of Technology of Curitiba – UNIFATEC, Paraná, Brazil.
2Collaborating Medicinal Biomagnetism Therapist, Brusque-SC.
3Co-supervising Professor Program in Biomagnetism and Bioenergy Applied to Health, Par Magnético Institute – IPM / University Center of Technology of Curitiba – UNIFATEC, Paraná, Brazil.
4Advising Professor Program in Biomagnetism and Bioenergy Applied to Health, Par Magnético Institute – IPM / University Center of Technology of Curitiba – UNIFATEC, Paraná, Brazil.
