REGISTRO DOI: 10.5281/zenodo.10373018
Tatiane Lobato da Silva1,
Apio Ricardo Nazareth Dias1,
Juarez Antônio Simões Quaresma1,3°†
Luiz Fábio Magno Falcão1,2°†*
Abstract: Hepatitis C virus (HCV) has a high genetic diversity, with seven genotypes with 86 sub types. This genetic variability confers persistence in the infection and escape of the immune system with evolution to cirrhosis and cancer. Environmental factors can contribute to different disease progression, being essential to assess the viral genotype in the infection and discuss the environ mental particularities of Eastern Amazon, and the frequencies of Liver fibrosis between different HCV genotypes in patients living in a region of the Brazilian Eastern Brazilian Amazon. Consists in an observational cross-sectional study. Sociodemographic and clinical data of 76 individuals diag nosed with Hepatitis C between 2019 and 2020 in public health services were selected. Data collected was tabulated in Microsoft Excel 2010TM spreadsheets and analysed in GraphPad Prism 5.0TM. Liver fibrosis was associated with genetic subtypes. Subtype 1b was predominant (42.1%), followed by 1a (13%) and 3a (1.3%). 69.7% of participants had chronic hepatitis, with mild fibrosis (F1/F2) being the most prevalent (38.1%). Severe fibrosis was detected in 75% of individuals infected with the subtype 1b, that is associated with more severe disease. We suggest further studies, to assess other commu nities in the region, as well as the monitoring of these patients with Liver Elastography to determine the disease evolution and its better management.
Keywords: Hepatitis C virus; Genetic; Chronic Hepatitis C; Liver; Elastography
1. Introduction
Hepatitis C virus (HCV) is a virus with a global infection incidence of 70 million worldwide (Mbituyumuremyi et al., 2018). Brazil’s northern region shows highest preva lence rate of this disease, and the states of Pará (Eastern Amazon) and Acre (Western Am azon) have the highest rates of this region, with 2% and 5.9%, respectively (Fonseca et al., 2004). Complications will be developed by 55 – 85% of infected people. Liver Cirrhosis and hepatocellular carcinoma were responsible for approximately 290,000 deaths associated with the disease in the year of 2019 (Piselli et al., 2021; WHO, 2022; Brazil Ministry of Health, 2021). Chronic HCV is one of the main health problems globally, with highest rate of complications in comparison to other viral hepatitis (Westbrook et al., 2014; Motawi et al., 2021; Yousaf et al., 2021).
HCV exhibits a high degree of genetic diversity, greater than HIV-1, being a challenge to development of vaccines and therapeutic by drugs (Messina et al., 2015). There are seven genotypes being described, subdivided into 86 subtypes. This genetic variability provides viral persistence, its mechanisms of immune escape, and increase the risk of evo lution to cirrhosis and cancer. The determination of the virus genotype is important be cause it is related to clinical presentation and prognostic (Wang et al., 2019; Strauss et al., 2013; Cheng et al., 2021). The most prevalent HCV genotypes in Amazon region are related with development of Liver fibrosis that are associated with disease complications.
The Amazon population is exposed to environmental factors that would influence the diseases evolution. The effects of rainforest fires or agrobusiness actions directly im pact in ecosystem structure, favouring the endemic diseases (Codeco et al., 2021); vulner ability of populations with lack access of health services, basic sanitation, and adequate nutrition (Alves de Oliveira et al., 2021); and genetic factors related to miscegenation of brazilian population must be considered. There is epidemiological evidence that the gen otype of this virus associated with these environmental, and individual behaviours influence in the disease progression (Azhar et al., 2020).
There are few studies associating clinical data of Liver Fibrosis with the viral genotypic classification in Eastern Amazon. This study aimed to identify the degree of liver fibrosis and HCV genotypes in patients from a community located in the Eastern Brazilian Amazon.
2. Materials and Methods
The study was approved by the Research Ethics Committee of Biologic and Health Centre, State of Pará University (opinion nº 4.478,618), being conducted in accordance with Helsinki Declaration for Research with human-beings, the data of participants was accessed from databases after authorization of Health authorities.
This was an observational cross-sectional study with quantitative, descriptive, and analytical characteristics. The sample consisted of patients residing in the city of Abae tetuba, Pará, Eastern Brazilian Amazon, evaluated in the public health services between years 2019 and 2020. Data were collected from 103 patients aged ≥ 18 years. Twenty-seven patients were excluded from the study due to non-performance of viral load and/or gen otyping tests (n=24) and co-infection (n=3): Human Immunodeficience Virus (HIV) (n=1) and Hepatitis B Virus (HBV) (n=2); resulting in a final sample of 76 clinical records ana lysed.
The clinical and epidemiological data were extracted from the patients’ clinical rec ords of viral hepatitis investigation from Notifiable Diseases Notification System (SINAN) and Sexual transmitted infectious diseases department (CTA/SAE). This public database are feed by health services when a diagnosis of a notifiable disease (e.g. HIV, HCV, tuber culosis, etc) was done, and contains (i) the Notification Form for viral hepatitis which con tains the sociodemographic data, (ii) the results of viral load tests, (iii) the results of viral genotyping, and (iv) the report of Liver Elastography exam (FibroscanTM, Echosens SA, Paris, France).
Sociodemographic data includes age, sex, color/race, schooling, occupation, municipality of residence, area of residence (urban or rural), marital status, possible form of transmission, clinical form. The genotype of HCV for diagnosis was determined by kit based assays which employ complementary probes to report genotype. This kit-based as says are easy-to-use and do not require expertise as is required for sequencing. HCV gen otype cobasTM HCV GT, manufactured by Roche (Roche, USA) claims to identify HCV genotypes 1-6 and can discriminate between subtype a and b of genotype 1.
The FibroscanTM transient elastography consists in a non-invasive exam that evalu ates the hepatic parenchyma at 25-65 mm of the skin in the right lobe of the liver. An ultrasound transducer in the axis of a vibrator is positioned in the same intercostal space where the liver biopsy is performed. Vibrations of mild amplitude and low frequency are transmitted from the vibrator to the tissues, inducing an elastic shear wave that propa gates through the tissues. Pulse-echo ultrasound acquisitions allow the propagation of the shear wave to be followed and its velocity to be measured, when stiffer the tissue, faster the shear wave is propagated. The patients were classified in the following fibrosis stages:
F0 < 7.1 kPa (normal liver), F1 (mild fibrosis), F2 (mild to moderate fibrosis), F3 (severe fibrosis), and F4 (cirrhosis) (de Lédinghen & Vergniol, 2008; Carnauba et al., 2018; Franco et al., 2019; Lutz et al., 2012).
The data was organized in Microsoft Excel 2010TM (Microsoft Software Inc., Rich mond, USA) spreadsheets and analysed using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, USA). Data with normal distribution were analysed using Stu dent’s T-test, while data without normal distribution were evaluated using Chi square or Fisher’s exact test. An alpha-level of 0.05 was adopted to reject the null hypothesis.
3. Results
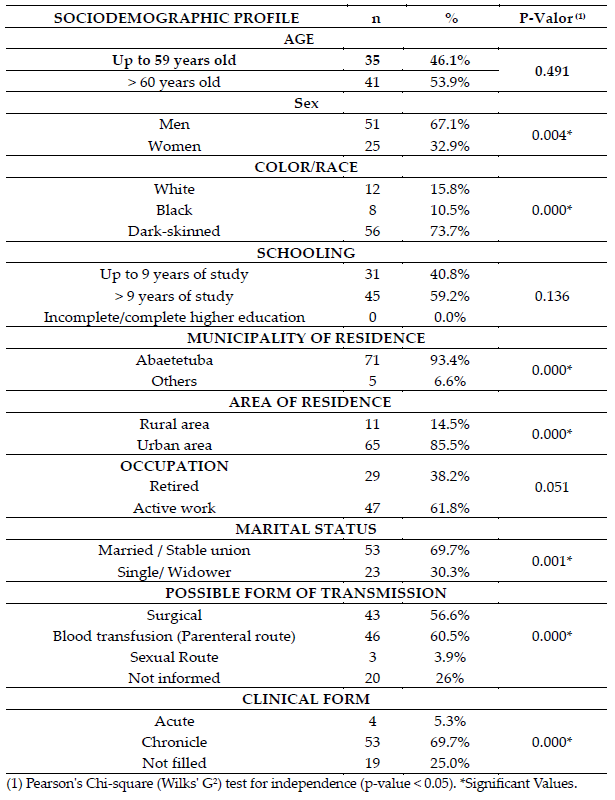
Sociodemographic profile of study participants shows a predominance of male pa tients (67.1%) and those aged ≥ 60 years (53.9%), who are married or in stable union (69.7%), self-declared Dark-skinned (73.7%), residing in the urban area (85.5%). with more than nine years of education (59.2%) (Table 1).
Table 1. – Sociodemographic profile of patients with hepatitis C, Abaetetuba, Pará, Brazil (2019- 2020).
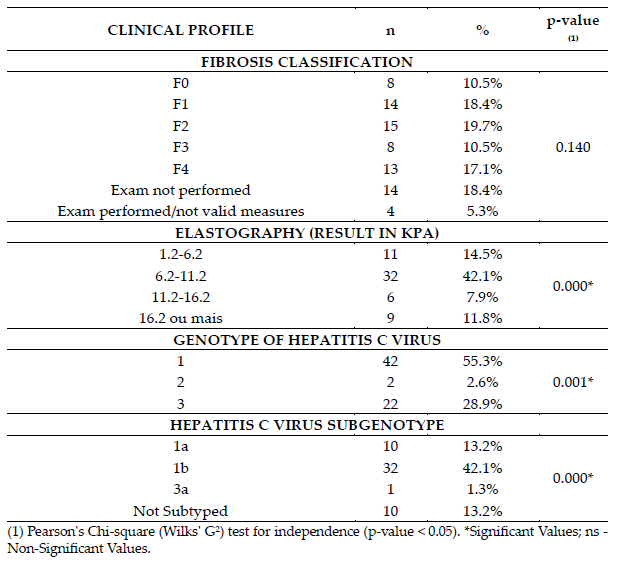
Clinical profiles of the cohort indicated that the degree of fibrosis measured were classified as mild /mild to moderate fibrosis in 38.1% of cases (F1[14, 18.4%] /F2[15, 19.7%]). There are a high prevalence of fibrosis degree classified as cirrhosis [F4(13, 17.1%)]. The most prevalent genotype was genotype 1 (55.3%), followed by genotype 3 (28.9%). The most expressive sub genotype was 1b (42.1%) (Table 2).
Table 2. – Clinical profile of patients with hepatitis C according to the degree of liver fibrosis and the genotype of the hepatitis C virus, Abaetetuba, Pará, Brazil (2019-2020).
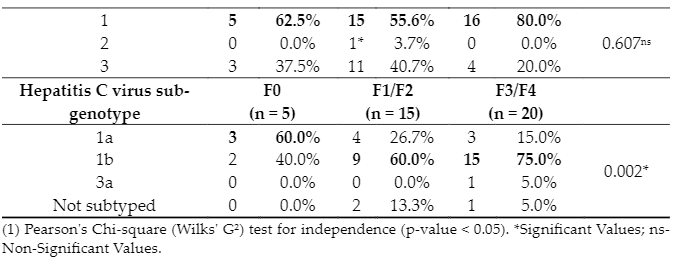
Genotypes and sub genotypes of the participants were associated with the degree of fibrosis. There is a difference between the different degrees of fibrosis related to their sub genotypes. Participants without fibrosis had type 1a sub genotype predominantly (3, 60%), while those with severe fibrosis (F3)/ cirrhosis (F4) (15, 75%), and mild (F1)/ mild to moderate (F2) fibrosis (9, 60%) had 1b sub genotype (Figure). There was no significant difference in the genotype of patients according to the different degrees of fibrosis; thus, patients without fibrosis (F0) (5; 62.5%), with mild (F1) / mild to moderate (F2) fibrosis (15; 55.6%), and with severe fibrosis (F3) / cirrhosis (F4) (16; 80%) had mostly type 1 genotype, between individuals infected with subtype 1b (15; 75%) are diagnosed with severe fibrosis (F3)/ cirrhosis (F4) (Table 3).
Table 3. – Distribution of patients with hepatitis C according to the genotypes, sub-genotypes, and liver elastography test (Kpa), Abaetetuba, Pará, Brazil (2019-2020).

4. Discussion
In this study, most individuals with HCV were male and aged ≥ 60 years. 59.2% of the participants had 9 years or more of education. There was a predominance of dark skinned (73.7%). With regard to transmission, 60.5% of patients stated that it occurred by blood transfusion. Genotype 1 had the highest prevalence rate (55.3%), type 3 was the second most prevalent type (28.9%), followed by type 2 (2.6%). There is a high frequency of individuals diagnosed with severe fibrosis or cirrhosis infected with subtype 1b (15; 75%).
Men are more vulnerable to the risks of hepatitis C, their biological, social, behav ioural, and cultural issues, with turn them more vulnerable to HCV infection. They have a greater intensity of sexual activity and seek for health services with less frequency than women (Nicolau et al., 2017). In a 2014 study, 50% of the patients had a complete high school education, which was not protective in terms of preventing HCV infection (Moia et al., 2014).
The genetic heterogeneity of population may impact the frequency and distribution of polymorphisms (Moia et al., 2014). Latin American population was composed by native americans, europeans, mostly from Spain and Portugal, and africans, that conferred a pre dominance of dark-skinned or black people in Brazilian and Amazonian population (Sua rez-Kurtz et al., 2014). About 30 million people are living in Amazon region, including quilombolas, indigenous people and riverine communities, as well as people of urban cen ters (Codeco et al., 2021). In this region, several people do not have adequate housing and basic sanitation conditions, and access to health services and adequate nutrition (Alves de Oliveira et al., 2021), It creates the conditions to maintenance and spread of infectious dis eases, that can potentially modify the development of HCV infection.
The majority of study participants developed the chronic form of the disease. It is known that infection occurs mainly through contact with contaminated blood. Transfu sion of blood and blood products was the main risk factor for hepatitis C transmission in the past, which resulted in a 10% risk of acquiring infection (Sá et al., 2013; WHO, 2022) , and the chronic form of the disease are the main clinical form of cases of hepatitis C, with > 60% of cases in almost all age groups analysed. Furthermore, another factor that may be related to the chronicity of the disease is the occurrence in people with less access to in formation and health services, as this vulnerability is a risk factor for infection and not early diagnosis (Salzano & Sans, 2014).
Viral hepatitis is a serious health problem in the Amazon. The earliest observations recognizing this, were made in the endemic areas of northwestern Colombia and western Brazilian Amazon (Benshabat & Dias, 1987). In the late 1960s, an outbreak of acute fulmi nating hepatitis occurred among Yanomami from the upper Orinoco river basin (Torres & Mondolfi, 1991). A similar outbreak occurred among Yukpa Ameridians by the end of 1970s, with 149 cases and 34 deaths (Hadler et al., 1984). These endemic or epidemic situ ations was the high frequency of cases with haemorrhagic manifestations, similar to an atypical form of yellow fever, leading to diagnostic error. Further research realized that the forest areas in the North and Center of South America were highly endemic for hepa titis agents (Echevarria & Leon, 2003).
The environmental and socioeconomic reality of Amazon region, linked to agrarian economy of region have an influence in evolution of infectious diseases (Codeco et al., 2021). The contamination of water, soil, air, and food by mining activity, deforestation, and rainforest fires causes loss of biodiversity, alteration in the structure and function of ecosystem. The global climate changes and Amazon rainforest savannization affects the health conditions of populations, exposing more than six million people to conditions of extreme risk for human health, 50% of these people live under conditions of high social vulnerability (Oliveira et al., 2021).
These climate changes impacts in the behaviour of disease-transmitting vectors (Oliveira et al., 2021). The interaction of population with other endemic infectious diseases, such as Malaria, Dengue, Zika, Chikungunya, Yellow Fever, Chagas Disease, HIV, HTLV, and others, as well as the relationship between these pathogens and HCV would be related with the disease evolution in this population (Figure).
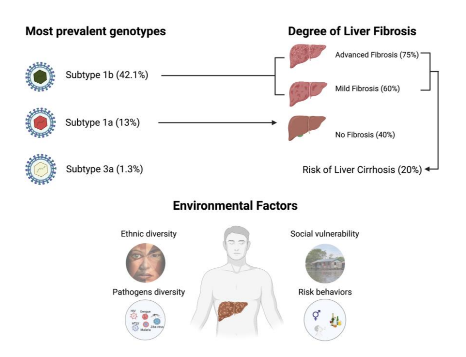
Figure 1. b, 1a and 3a are the most prevalent in Eastern Amazon, Subtype 1b is related to Advanced and Mild Liver fibrosis, the individuals with fibrosis have a risk to development of Liver cirrhosis of 20%. Associated to HCV infection, the environmental factors as ethnic diversity, social vulnera bility, diversity of pathogens in Amazon region, and the risk behaviors increase the risk of worse prognostic.
In this study Genotype 1 had the highest prevalence rate (55.3%). Type 3 was the second most prevalent type (28.9%), followed by type 2 (2.6%). This result corroborates other studies conducted in the Brazilian Amazon and Latin American countries. Regard ing sub-genotypes, the most prevalent subtype was 1b (42.1%). Studies carried out in other locations corroborate the results of this research, in the Eastern Amazon (Moia et al., 2014; Confalioneri et al., 2013; Sawada et al., 2011; Guimaraes et al., 2018), Paraguay and Co lombia (Echevarria & Leon, 2003; Oliveira et al., 2021), and recent studies from Northern, Southern and Eastern Europe have also shown the predominance of genotype 1(Confa lonieri et al., 2014; Sawada et al., 2011; Guimarães et al., 2018).
Studies of genotypic distribution is important for a better understanding of the epi demiological profile, and the clinical diagnostic, and therapeutic measures to be adopted in which case. The prognosis, duration of treatment, cure rates and drugs to be utilized depends in great measure of the genotype and subtypes. Genotype 1 is responsible for more cases than any other, worldwide, with about 83.4 million cases, Genotype 3 is the second most common, with 54.3 million cases estimated (Messina et al., 2015), and the subtypes 1b and 3a are associated with more severe forms of the disease, fibrosis, and a major risk for development of hepatocellular carcinoma (Barbosa et al., 2019; Messina et al., 2015; Olmedo et al., 2019).
The degree of fibrosis measured were severe fibrosis/cirrhosis (F3/F4) in 80% of the Individuals infected with HCV genotype 1, as well as, 75% of the infected with subtype 1b. It is known that the weighting of the fibrotic degree of the liver in patients with hepa titis C has a high value in terms of diagnosis and prognosis, since approximately 20% of patients with chronic disease can have progression to liver cirrhosis (Bruno et al., 2007). Liver fibrosis grade according to the liver elastography test, which is a non-invasive, validated diagnostic method used to identify and classify liver fibrosis, was classified as follows. F0 (without fibrosis) < 7.1 kPa; F1/F2 (mild/ mild to moderate fibrosis) 8.8–9.5; and F3/F4 (severe fibrosis/ cirrhosis) 9.6 to ≥ 12.5 (Carnauba et al., 2018; Franco et al., 2019; Lutz et al., 2012).
However, 18.4% (n=14) of the participants did not undergo an examination to verify the degree of fibrosis. These data are worrying, as it is of fundamental importance to de termine the degree of fibrosis so that the patient can receive the suitable therapeutic measures and the most appropriate follow-up for their clinical condition. It is known that, even today, the gold standard for this determination is liver biopsy; and despite Liver transient elastography be an efficient non-invasive technique, have a high cost and is less available in public health centres (Franco et al., 2019; Fahmy et al., 2011; Mello et al., 2020).
5. Conclusions
The study revealed the high predominance of genotypes 1 and 3 and subtypes 1a, 1b, and 3a among the studied population, with a higher prevalence of severe liver fibrosis (F3) and cirrhosis (F4) between individuals infected with genotype 1, subtype 1b. These HCV genotypes are the most prevalent in the national territory, being associated with more aggressive clinical forms. The results of this study allow us to elucidate the geno typic behaviour of HCV and its association with liver fibrosis and cirrhosis in the Brazilian Eastern Amazon, which is known to be a region of high prevalence for the virus, and with environmental factors that would impacts in the evolution of diseases.
Author Contributions: Conceptualization, TLS and LFMF; methodology, LFMF and JASQ; valida tion, TLS, ARND, JASQ and LFMF; formal analysis, LFMF; investigation, TLS; resources, JASQ; data curation, ARND and LFMF; writing—original draft preparation, TLS; writing—review and editing, ARND; visualization, LFMF.; supervision, LFMF; project administration, LFMF; funding acquisition, JASQ. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding. The APC was funded by Programa de Apoio a Publicação Qalificada (PROPESP/UFPA).
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Biologic and Health Centre, State of Pará University (protocol code 4.478,618)
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: The research data could be obtained from Correspondent author.
Acknowledgments: The authors must thanks to all participants of study.
Conflicts of Interest: The authors declare no conflict of interest.
References
1. Mbituyumuremyi A, Van Nuil JI, Umuhire J et al. Controlling hepatitis C in Rwanda: a framework for a national response. Bull World Health Organ. 2018;96(1):51-8.
2. Fonseca JCF, Brazil LM. Hepatitis C virus infection in the Brazilian Amazon region. Rev. Soc. Bras. Med Trop. 2004; 37: 1-8. 3. Piselli P, Serraino D, Fusco M et al. Hepatitis C virus infection and risk of liver-related and non-liver-related deaths: a popula tion-based cohort study in Naples, southern Italy. BMC Infect Dis. 2021; 21:667. https://doi.org/10.1186/s12879-021-06336-9 4. World Health Organization, Hepatitis C, Hepatitis C key facts, World Health Organization Media Centre, Geneva, Switzerland, 2022, https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
5. Brazil Ministry of Health, 2021 Epidemiological Bulletin on Viral Hepatitis, Health Surveillance Secretariat, Department of STD, AIDS and Viral Hepatitis, Ministry of Health, Brasília, Brazil, 2021 (Portuguese), http://www.aids.gov.br/pt-br/pub/2021/bo letim-epidemiologico-hepatites-virais-2021
6. Westbrook RH, Dusheiko G. “Natural History of Hepatitis C.” Journal of Hepatology. 2014; 61: S58-68. 7. Motawi TMK, Sabry D, Shehata NI, et al. Impact of FOXP1 rs2687201 genetic variant on the susceptibility to HCV-related hepa tocellular carcinoma in Egyptians. J Biochem Mol Toxicol. 2021;16: e22965.
8. Yousaf A, Ghafoor A, Fátima N, et al. Gender-specific frequency distribution of hepatitis C virus genotypes in Punjab Province, Pakistan: a clinically significant cross-sectional study. Cureus. 2021; 13: e17480.
9. Wang B, Krüger L, Machnowska P, et al. Characterization of a hepatitis C virus genotype 1 divergent isolate from an HIV-1 coinfected individual in Germany assigned to a new subtype 1o. Virol J. 2019;16: 2803-04.
10. Strauss E. Natural History of Hepatitis C – Progression Factors. Prognostic assessment of chronic hepatitis C. Treatise on Viral Hepatitis and Associated Diseases. São Paulo: Atheneu. 2013: 453-470.
11. Codeço CT, Dal’Asta AP, Rorato AC. et al. Epidemiology, Biodiversity, and Technological Trajectories in the Brazilian Amazon: From Malaria to COVID-19. Front Public Health. 2021; 13: 647754. doi: 10.3389/fpubh.2021.647754.
12. Alves de Oliveira, BF, Bottino, MJ, Nobre, P. et al. Deforestation and climate change are projected to increase heat stress risk in the Brazilian Amazon. Commun Earth Environ. 2021; 2. Doi: 10.1038/s43247-021-00275-8
13. Azhar MJ, Khalid N, Azhar S, et al. Study of the Effect of Different Hepatitis C Virus Genotypes on Splenomegaly. Cureus. 2020; 3112: e10164.
14. de Lédinghen V, Vergniol J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008 Sep;32(6 Suppl 1):58-67. doi: 10.1016/S0399-8320(08)73994-0. PMID: 18973847.
15. Nicolau S, Medeiros AS, Santos MCA, Montarroyos JS. Epidemiological profile of hepatitis B in a regional health center in Recife, Rev. Saúde Col. UEFS. 2017; 7: 30-35.
16. Moia LJMP, Amaral ISA, Farias AJL, Silva MMA. Hepatitis C virus infection in a referral hospital in the Brazilian Amazon. Rev Para Med. 2014; 28:19-25
17. Suarez-Kurtz G, Paula DP, Struchiner CJ. Pharmacogenomic implications of population admixture: Brazil as a model case. Pharmacogenomics. 2014; 15: 209-19. doi: 10.2217/pgs.13.238.
18. Salzano FM and Sans M. Interethnic admixture and the evolution of Latin American populations. Genetics and Molecular Biol ogy [online]. 2014; 37 suppl 1:151-170. Doi:10.1590/S1415-47572014000200003.
19. Sá LC, Araújo TME, Griep RH, Campelo V, Monteiro CFS. Hepatitis C seroprevalence and associated factors in crack users. Rev Latinoam Enferm. 2013; 21:1195-202.
20. Bensabath, G & Dias, LB. Hepatitis Labrea e outras hepatites fulminantes em Sena Madureira, Acre e Boca do Acre, Amazonas, Brasil. Revista do Instituto de Medicina Tropical de São Paulo. 1987; 25:182-194.
21. Torres JR & Mondolfi, A. Protracted outbreak of severe delta hepatitis: Experience in an Amerindian population of the Upper Orinoco Basin. Reviews of Infectious Diseases. 1991; 13:52-55.
22. Hadler SC, Monzon M, Ponzetto A, Anzola E, Rivero D, Mondolfi A, Bracho A, Francis DP, Gerber MA, Thung S, Gerin J, Maynard JE, Popper H, Purcell RH. Delta virus infection and severe hepatitis. An epidemic in the Yucpa indians of Venezuela. Annals of Internal Medicine. 1984; 100:339-344.
23. Echevarría JM, León P. Epidemiology of viruses causing chronic hepatitis among populations from the Amazon Basin and related ecosystems. Cad Saude Publica. 2003 Nov-Dec;19(6):1583-91. doi: 10.1590/s0102-311×2003000600003. 24. Oliveira BFA, Bottino MJ, Nobre P, Nobre CA. Deforestation and climate changes are projected to increase heat stress risk in the Brazilian Amazon. Communications, Earth & Environment. 2021; 12:207. Doi: https://doi.org/10.1038/s43247-021-00275-8 25. Confalonieri UEC, Margonari C, Quintão AF. Environmental change and the dynamics of parasitic diseases in the Amazon. Acta Tropica. 2014; 129: 33-41. Doi:10.1016/j.actatropica.2013.09.013.
26. Sawada L, Pinheiro ACC, Locks D, et al. Distribution of hepatitis C virus genotypes among different exposure categories in the State of Pará, Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2011;44: 8-12.
27. Guimaraes VS, Melo TG, Ferreira RCD, Almeida SF, Martins LC. Prevalence of hepatitis C virus genotypes in the State of Pará, Brazil. Rev. Soc. Bras. Med. Trop. Uberaba, 2018; 51: 508-512.
28. Barbosa KMV, Moreira LVL, Oliveira CMA, et al. Hepatitis C in the 1980s: rescue of old “non-A and non-B” hepatitis cases from a hepatology service in the Amazon, Brazil. 2019. Rev Pan Amaz Saude, 2019; 10:1-11.
29. Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015; 61: 77-87.
30. Olmedo G, Nagai M, Sugastti AL, et al. Epidemiological and molecular characterization of Hepatitis C in the population that went to the Central Laboratory of Public Health, Paraguay 2013-2018. Rev. public salud Parag. 2019; 9: 73-80. 31. Santos Ó, Gómez A, Vizcaíno V, Casas MC, Ramírez MDP, Olaya P. [Hepatitis C virus genotypes circulating in Colombia]. Biomedica. 2017 Jan 24; 37:22-27.
32. Petruzziello A, Sabatino R, Loquercio G, et al. Nine-year distribution pattern of hepatitis C virus (HCV) genotypes in Southern Italy. PLoS One. 2019; 14: e0212033.
33. Bruno S, Crosignani A, Maisonneuve P, Rossi S, Silini E, Mondelli MU. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology. 2007 Nov;46(5):1350-6. doi: 10.1002/hep.21826. PMID: 17680653.
34. Kermani FR, Sharifi Z, Ferdowsian F, Paz Z, Zamanian M. Distribution of hepatitis C virus genotypes among chronic infected injecting drug users in Tehran, Iran. Jundishapur J Microbiol. 2013; 6: 265-8.
35. Carnaúba LAB, Pontes LR, Flavio LF, et al. Hepatitis C staging: elastography compared with liver biopsy. Cad. Med. UNIFESO. 2018; 1(1): 111-123.
36. Franco, KMV, Vieira WB, Dias ARN, et al. Doppler ultrasound: a non-invasive method used to diagnose and monitor patients with chronic hepatitis C. Journal of Gastroenterology and Hepatology. 2019; 35.
37. Lutz H, Gassler N, Tischendorf F, Trautwein C, Tischendorf J. Doppler ultrasound of liver blood flow for noninvasive assess ment of liver fibrosis compared with liver biopsy and transient elastography. Excavation. Dis. Sci. 2012; 57: 2222–30.
38. Fahmy MI, Badran HM. Comparison of transient elastography with Doppler indices in predicting hepatitis C-induced liver fibrosis and cirrhosis. The Egyptian J. Radio. Nucl. Med. 2011; 42:111–7.
39. Mello FSF, Lima JMDC, Hyppolito EB, et al. Comparison of hepatic fibrosis degrees in chronic hepatitis C (HCC) measured by elastography and serology methods: ARFI and Fibroscanvs APRI and FIB4. Rev. Med. UFC. 2020; 60: 18-25.
1Health and Biological Centre, State of Pará University, 2623 Perebebui St, Belem, Pará 66087-670, Brazil;
2University of São Paulo, 455 Dr Arnaldo Ave, São Paulo, São Paulo 01246-903, Brazil;
3Tropical Medicine Centre, Federal University of Pará, 92 Generalissimo Deodoro Ave, Belém, Pará 66055-240, Brazil;
° These authors contributed equally for this study;
† These authors share Senior authorship;
* Correspondence: fabiofalcao@uepa.br
