REGISTRO DOI:10.5281/zenodo.12680467
DVM, MS Marcelo B. Santos-Junior 1
DVM Scarlath O P Santos2
DVM, MS Tainara M. B. Peixoto1
MS, DVMLuciana M Mello1
DM, MSPaula G. A. Cabral1
DVM Isabella C. Morales1
DVM, MS, PhdAndré L. A. Oliveira3*
Abstract
Objective: To improve the surgical technique for implanting an extraluminal intrathoracic prosthesis made from biocompatible material (Nitinol).Experimental design: Twelve healthy New Zealand rabbits were used and randomly subdivided into two experimental groups (G1 – SHAM group; G2 – operated group). All animals underwent surgery following identical technical standards and were evaluated using tomographic examinations immediately postoperatively and 14 days after the procedure. On the 14th day, after tomography, all animals were euthanized and macroscopically evaluated during necropsy.Animals: Twelve healthy female New Zealand rabbits were included, subdivided into 2 experimental groups with 6 rabbits in each group (G1: SHAM group, G2: prosthesis group).Methods: All animals underwent a surgical procedure involving exposure, tracheal dissection, and implantation of an extraluminal prosthesis. They were evaluated immediately postoperatively and on day 14 using computed tomography.Results: The evaluation period was 14 days. Complications such as death, laryngeal paralysis, and pulmonary edema were observed in some animals from both G1 and G2. The mortality rate ranged from 0.33 to 0.5 with a p-value of 0.5732 between the groups; for laryngeal paralysis, it was 0.1667 with a p-value of 1. Pulmonary edema was observed in only one animal.Conclusion: The implantation of a new intrathoracic extraluminal helical prosthesis was found to be satisfactory, provided appropriate precautions are taken during the procedure.
INTRODUCTION
Tracheal collapse (TC) is a degenerative and progressive disease affecting the trachea, primarily seen in middle-aged to elderly pets. The exact pathophysiological mechanism remains unclear, but some studies indicate degeneration of tracheal rings with reduced glycosaminoglycans and hypocellularity. These changes result in increased tracheal cartilage flaccidity and dorsal membrane laxity, leading to dynamic tracheal collapse 1.
Small dog breeds, such as Shih Tzu, Chihuahua, Maltese, Pomeranian, Yorkshire Terrier, and Miniature Pinscher, are predominantly affected. Additionally, clinical signs are more prevalent in obese and older patients 1,2. Diagnosis primarily relies on clinical signs including dry, harsh cough, dyspnea, exercise intolerance, cyanosis, and syncope. Coughing worsens during excitement or with neck compression, exacerbating tracheal stimulation 3-6.
Classification based on CT findings divides TC into four stages: Stage 1 (up to 25% reduction in tracheal lumen), Stage 2 (25-50% reduction), Stage 3 (50-75% reduction), and Stage 4 (greater than 75% reduction) 4.
Current drug therapies aim to reduce respiratory distress and improve quality of life, utilizing corticosteroids, antitussives, bronchodilators, antibiotics for secondary infections, and occasionally androgenic steroids like stanozolol 1,7,8.
Patients unresponsive to medical therapy or in advanced stages (3 and 4) often require surgical intervention. Various techniques have been proposed, including intraluminal and extraluminal prostheses 9-11. However, consensus on the optimal surgical approach remains elusive, with reported complications necessitating intensive postoperative care and impacting patient outcomes.
Therefore, this study aims to establish a surgical technique for intrathoracic tracheal prosthesis implantation using biocompatible materials, aiming to minimize intra- and postoperative complications. Given the prevalence of TC in companion animals, improving its management is crucial for enhancing patient quality of life and survival.
MATERIALS AND METHODS
The experimental protocol for improving the technique of helical extraluminal prosthesis implantation for tracheal collapse correction was submitted, analyzed, and approved by the Ethics Committee on the Use of Animals (CEUA) of Universidade Estadual do Norte Fluminense Darcy Ribeiro – UENF under protocol number XXXXX. The study was conducted using 12 New Zealand rabbits (Oryctolagus cuniculus) from the experimental vivarium of the Animal Experimentation Unit (UEA-UENF).
All selected rabbits were adult females weighing between 2.74 and 4.2 kg, confirmed healthy through prior clinical evaluation, and acclimatized individually in wire cages. After random selection, the rabbits were equally divided into two experimental groups: Group 1 (sham operated, SHAM) and Group 2 (operated).
Initially, all animals received pre-anesthetic medication (MPA) consisting of ketamine (40 mg/kg) and xylazine hydrochloride (5 mg/kg), administered intramuscularly. Anesthesia induction was achieved with intravenous propofol (5 mg/kg), followed by maintenance with isoflurane (Isoflurane, BioChimico®, Rio de Janeiro – RJ, Brazil) at 2-3.5% v/v with oxygen (2 L/min) via inhalation mask throughout the surgical procedure. Isoflurane administration was discontinued at the end of surgery, and animals were maintained on oxygen until complete recovery from anesthesia.
Following proper trichotomy and confirmation of anesthesia depth, the rabbits were positioned in dorsal recumbency with a folded drape placed dorsally over the neck area to optimize exposure of the surgical field. After a skin and subcutaneous tissue incision, the sternohyoid and sternocephalic muscles were dissected to expose the trachea adequately, as depicted in Figure 1.
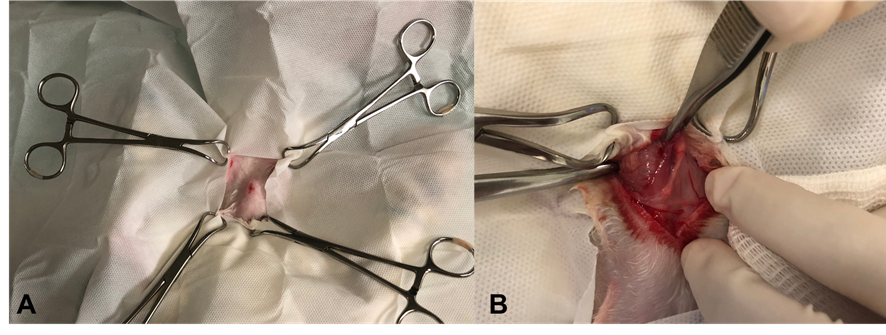
Figure 1 – Surgical technique for placing an extra luminal helical stent in a rabbit (InTrak®). A: Operating field. B: Visualization of the operative field after skin and subcutaneous incision and beginning of divulsion of muscle groups. Source: Personal Archive, 2019.
Once the trachea was identified, the peritracheal tissue was dissected using Metzenbaum scissors until the cervical trachea was completely exposed. Special care was taken throughout to avoid injury to the laryngorecurrent nerves or the trachea itself, as depicted in Figure 4A. After achieving proper tracheal exposure, a curved hemostatic forceps was used for careful cranial traction of the trachea to dissect the peritracheal tissue at the thoracic inlet. This was done with meticulous manipulation and accurate visualization of the adjacent structures to the trachea, as illustrated in Figure 2B. Finally, blind digital dissection was performed until the mediastinal region of the animals was palpated correctly.
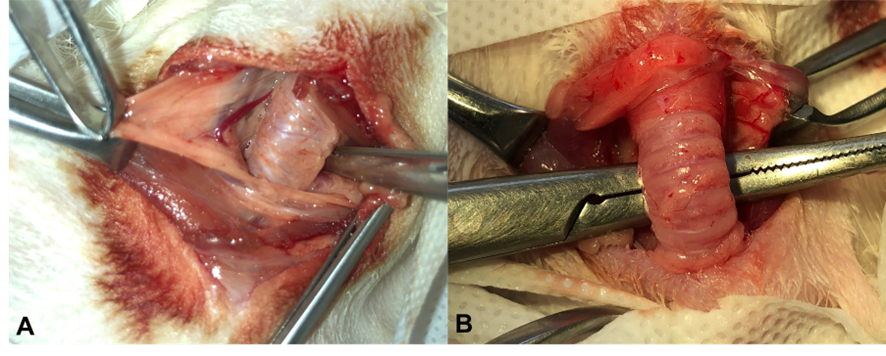
Figure 2: Visualization of the cervical trachea of rabbits. A: Dissection of peritracheal tissue. B: Cranial retraction of the trachea with the aid of a curved hemostatic forceps. Source: Personal archive, 2019.
After correct dissection and identification of the mediastinal region by the responsible surgeon, an extraluminal helical prosthesis made of Nitinol, 0.7 mm thick, 6 mm wide, and 42 mm long (InTrak® A7-0642-7V) was placed. Only the animals in Group 2 received the prosthesis.
The prostheses were all positioned starting from the third tracheal ring and were maneuvered in a circular motion in a caudal direction, as shown in Figure 3A. Once fully positioned around the trachea, they were carefully maneuvered into the chest cavity and securely fixed in the mediastinal region. For fixation, 4-0 polypropylene thread was used, creating two sutures around the tracheal ring and the prosthesis—one in a cranial region and one in a caudal region—to prevent displacement of the prosthesis within the mediastinal cavity.
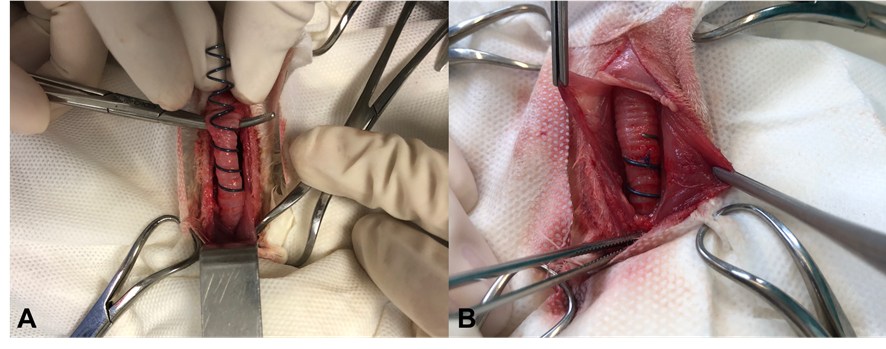
Figure 3: Placement of the helical nitinol prosthesis in a rabbit (InTrak®). A: placement of an extra luminal prosthesis in the cervical trachea in a caudal direction to the thorax entrance. B: Prosthesis properly positioned, detail for suture made around the tr acheal ring and the prosthesis, with 4-0 polypropylene suture. Source: Personal archive, 2019.
After the prosthesis was correctly fixed, muscular fascias of the sternohyoid and sternocleidomastoid muscles were sutured with a simple continuous technique. Subsequently, subcutaneous tissue was reduced using a Cushing pattern and the skin was closed with a simple continuous suture.
All animals in both groups received post-operative antimicrobial therapy with enrofloxacin at a dose of 8 mg/kg SID orally for 5 days. Analgesia was provided with dipyrone at 12 mg/kg BID for 5 days, tramadol at 5 mg/kg BID for 5 days, and ketoprofen at 1 mg/kg SID for 3 days, all administered orally.
Immediate and 14-day post-operative tomographic examinations were conducted to detect any surgery-related complications initially and late complications potentially caused by the prosthesis. The positioning and possible displacement of the prosthesis in Group 2 animals were also assessed. Tomographic images were reconstructed using Osirix Lite® v.11.0.2 and evaluated by a skilled diagnostic imaging observer.
Tracheal diameters were measured in three segments: cervical (second tracheal ring caudal to the larynx), thoracic entrance (level of the manubrium), and thoracic (cranial to the carina). Measurements were taken in both height and length at each time point of the experiment.
Additionally, in Group 2, the distance the prosthesis entered into the mediastinal region was measured, from the manubrium to the distal tip of the inserted prosthesis.
Animals were euthanized on the fourteenth day post-surgery after undergoing a final tomographic examination. Euthanasia involved premedication with intramuscular ketamine (40 mg/kg) and xylazine hydrochloride (5 mg/kg), followed by intravenous induction with propofol (5 mg/kg). After achieving a deep anesthetic plane, euthanasia was induced by intravenous administration of 5 mL of 19.1% potassium chloride. Necropsy was immediately performed to identify any anatomical changes, focusing on the neck, mediastinal, and thoracic regions, with particular attention to the trachea for macroscopic alterations.
STATISTICAL ANALYSIS
Complications observed during the experiment, such as death, laryngeal paralysis, pneumothorax, pulmonary granuloma, pneumonia, respiratory noise, and pulmonary edema, were evaluated. Additionally, experimental times including total anesthesia time, incision, tracheal dissection, prosthesis placement, and closure (raffia) were analyzed using generalized linear models (Tables 1 and 2) with a Binary distribution. The GLIMMIX procedure of SAS System software (SAS Institute Inc., Cary, NC, USA) was employed, and where significant differences were found, the Tukey test (5%) was applied.
Variables such as weight, diameters of the cervical trachea, intrathoracic trachea diameter, carina diameter, and distance from the prosthesis (Table 3) were evaluated using mixed models. The PROC MIXED procedure of SAS System software (SAS Institute Inc., Cary, NC, USA) was utilized, and significant differences were assessed using the Tukey test at a significance level of 5%.
RESULTS
During the experimental period, 25% of the animals (n=5) died postoperatively. Among these, two belonged to Group 1 (G1) and three belonged to Group 2 (G2). Although not all deaths could be attributed to specific causes, respiratory complications such as laryngeal paralysis, pneumonia, and pulmonary edema were noted. There was no statistical difference in the number of deaths between G1 and G2
Laryngeal paralysis was observed in 16.66% of the animals (n=2), with one case in G1 and one in G2. Both animals died, but at different times: the animal from G2 died on the ninth post-operative day, while the animal from G1 died on the fourth post-operative day. In this study, laryngeal paralysis resulted from recurrent laryngeal nerve rupture, as observed during necropsy, depicted in Figure 4.
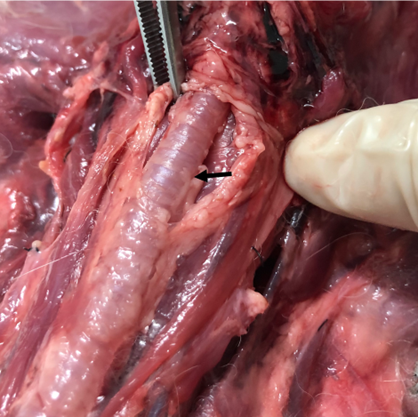
Figure 4: Image of trachea obtained during necropsy of the rabbit with clinical changes of laryngeal paralysis. Note disruption of the left recurrent laryngo nerve (arrow). Source: Personal Archive, 2019.
Another complication observed in the present study was the occurrence of pneumothorax, present in 25% of the animals evaluated (n=3). Of these, two animals belonged to G1 and one to G2. One of the animals in G1 also developed laryngeal paralysis and died due to complications; the other did not present any complications except pneumothorax. The animal in G2 also developed pneumonia and died due to complications.
The formation of pulmonary granuloma was observed in 16.66% of the animals tested (n=2), one in G1 and one in G2. In both cases, a sample was collected for culture and antibiogram analysis, and Staphylococcus intermedius and Staphylococcus sp. were isolated. In the antibiogram, the isolated microorganisms demonstrated weak resistance and were sensitive to most of the antibiotics tested, including enrofloxacin, an antibiotic used in the postoperative period for all animals in the present study. None of the animals in which granuloma was observed died, making it a necroscopic finding. The macroscopic visualization of the pulmonary granuloma can be seen in Figure 5.
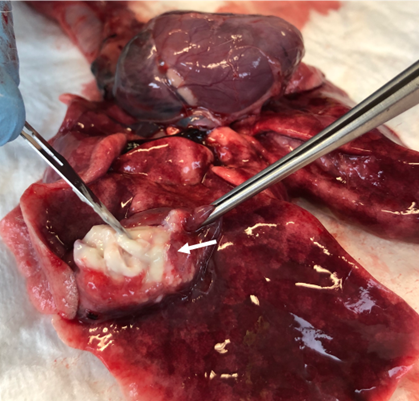
Figure 5:Lung image obtained during rabbit necropsy without the presence of clinical signs. Note pulmonary granuloma (arrow), observed in one of the G1 animals. In the present animal, Staphylococcus intermedius was isolated. Source: Personal archive, 2019.
One of the animals used in the experiment (8.33%) presented pneumonia; this animal belonged to G1 and died (Figure 6). Respiratory noises were present in 25% of the animals (n=3); one of these animals belonged to G1, and the other two to G2. The rabbit belonging to G1 and one of the rabbits belonging to G2, which presented respiratory noise, had laryngeal paralysis. The other animal in G2 also developed a pulmonary granuloma and pulmonary edema.
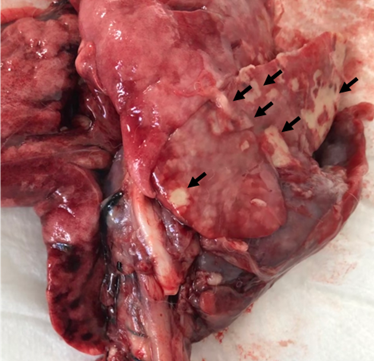
Figure 6: Lung image obtained during necropsy of the rabbit that died. Note the lung of a rabbit affected by bronchopneumonia (arrows). Source: Personal archive, 2019.
Only one animal belonging to G2 (8.33%) developed pulmonary edema (Figure 7); this same animal also developed a pulmonary granuloma and respiratory sounds.
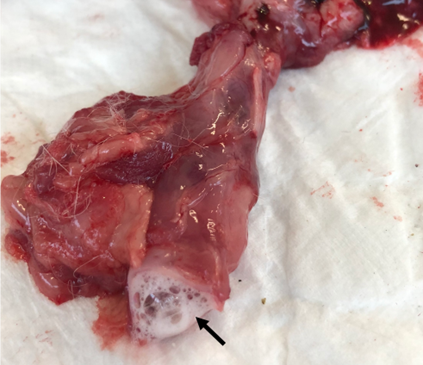
Figure 7: Image of larynx obtained during necropsy of an animal with respiratory sounds. Macroscopic visualization of foamy secretion in the respiratory tract of an animal affected by pulmonary edema (arrow). Source: personal archive, 2019.
As can be seen in Table 1, for the variables related to postoperative complications, there was no statistical difference between groups G1 and G2.
Table 1 – Main complications observed during the experiment.
Observed complications Groups Frequency Standard Error p value Death G1 0,3333 0,1925 0,5732 G2 0,5 0,2041 Laryngeal Paralysis G1 0,1667 0,1521 1 G2 0,1667 0,1521 Pneumotorax G1 0,3333 0,1925 0,5265 G2 0,1667 0,1521 Pulmonary granuloma G1 0,1667 0,1521 1 G2 0,1667 0,1521 Pneumonia G1 1,283.10-6 4,62 . 10-4 0,9742 G2 0,1667 0,1521 Respiratory Noise G1 0,1667 0,1521 0,5265 G2 0,3333 0,1925 Pulmonary Edema G1 1,283.10-6 4,62 .10-4 0,9742 G2 0,1667 0,1521
No statistical differences were observed in operative times (Table 2). However, it can be seen that from the first procedure to the last, there was a gradual decrease in operative time, which can be attributed to the surgeon’s learning curve. Therefore, the total surgery time varied between 8-25 minutes for G1 and 16-24 minutes for G2, with the longer time in G2 being due to the placement of the prosthesis, which was not done in G1.
The prosthesis placement time in G2 averaged 5.667 minutes, with times varying between 5-7 minutes. The incision time, from the beginning of the skin incision to the identification of the trachea, averaged 3.8333 minutes for G1 and 4.5 minutes for G2, with the time varying between 2-6 minutes for G1 and 3-9 minutes for G2. The dissection of the trachea, from the identification of the trachea and the start of dissection of the peritracheal tissue to the placement of the prosthesis in G2 or the beginning of the raft in G1, also showed no statistical difference, with an average of 4.8333 minutes in G1 and 6.1667 minutes in G2, with a variation of 3-7 minutes for G1 and 3-10 minutes for G2. Finally, the raffling time varied from 3-10 minutes in G1 and 3-5 minutes in G2, with the average time being 5 minutes for G1 and 4.1667 minutes for G2.
Table 2 – Operative times
Experimetal times Groups Frequency Standard Error p value Anesthesia Total Time G1 43,8333 4,8216 0,5033 G2 48,8333 5,3715 Incision G1 3,8333 0,6054 0,4892 G2 4,5 0,7107 Tracheal Dissection G1 4,8333 0,7132 0,7559 G2 5,1667 0,7624 Prosthesis placement G2 5,6667 0,2978 Suture G1 5 0,7113 0,3861 G2 4,1667 0,5928
During the experiment, we sought to perform tomographic examinations to identify possible respiratory complications inherent to the surgical procedure, as well as variations in tracheal diameters that could occur secondary to the presence of the prosthesis. In the cervical trachea, height and width measurements were obtained at the second tracheal ring, caudal to the larynx (Figure 8).
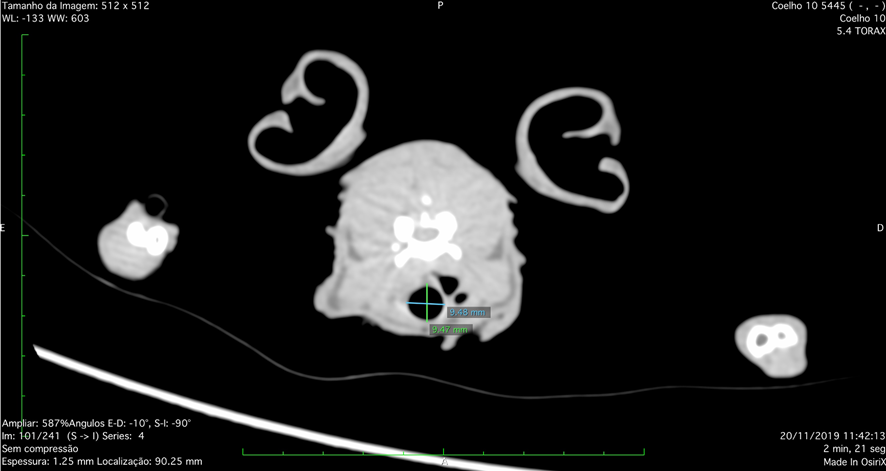
Figure 8: Tomographic image, frontal plane, of the upper respiratory tract obtained in a New Zealand rabbit. Measurements of the height (green line) and width (blue line) of the cervical trachea are noted, performed on the second tracheal ring, caudal to the larynx. Source: Personal archive, 2019.
When we took into account the height measured at the cervical rings, an average of 8.02mm was observed in G1 and 7.05mm in G2, with a significant difference between the groups (p=0.0399). When we analyzed the width of the tracheal rings, the average for G1 was 7.02mm and 5.86mm for G2, with a statistical difference between the groups (p=0.0016). At the entrance to the thorax, height and width measurements were also obtained for the tracheal rings, with the measurement of the tracheal ring at the height of the animals’ manubrium being standardized.
When we took into account the height measured in the intrathoracic rings at the entrance to the chest, an average of 6.79mm was observed in G1 and 4.66mm in G2, with a significant difference between the groups (p<0.0001). When we analyzed the width of the tracheal rings, the average for G1 was 7.00mm and 4.83mm for G2, with a statistical difference between the groups (p<0.0001). The intrathoracic trachea was measured at the level of the last tracheal ring cranial to the carina.
When we took into account the height measured in the intrathoracic rings cranial to the carina, an average of 6.13mm was observed in G1 and 5.84mm in G2, with no significant difference observed between the groups (p=0.5053). When we analyzed the width of the tracheal rings, the average for G1 was 7.00mm and 6.75mm for G2, with no statistical difference observed between the groups (p=0.6456).
Additionally, in Group 2, the distance to place the prosthesis was measured, that is, how far the prosthesis entered the mediastinal region. To do this, measurements were taken from the manubrium to the distal tip of the inserted prosthesis. The implantation distance of the helical prosthesis varied between 15.7-21.6mm initially (immediate postoperative period) and 18-24.9mm at the second measurement (fourteenth postoperative day). To identify the correct intrathoracic positioning of the nitinol prosthesis, three-dimensional reconstructions were performed in the sagittal plane (Figure 9).
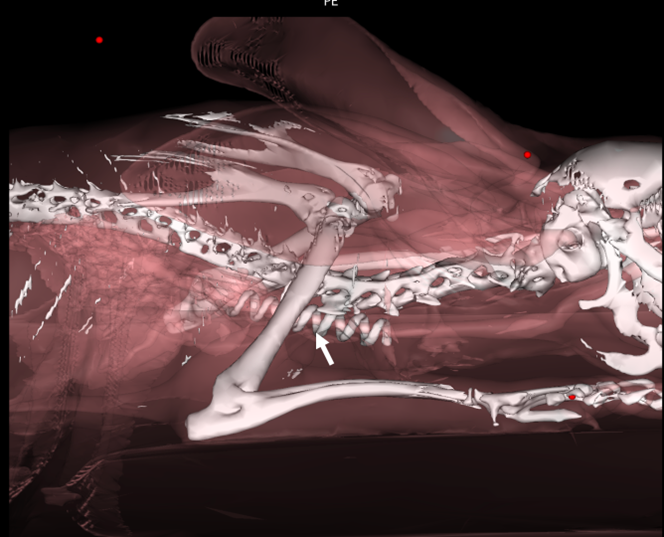
Figure 9: Three-dimensional volumetric reconstruction of 1.25mm thick tomographic images, obtained using the Osirix Lite® v.11.0.2 program. Note helical nitinol prosthesis implanted inside the thorax extraluminally (arrow). Source: Personal archive, 2019.
All parameters obtained from tomographic examinations and presented here can be seen in Table 3.
Table 3 – Diameters measured from tomographic examinations.
Experimental Time Groups Frequency Standard Error p value CTh Diameter G1 8,0233 0,303 0,0399 G2 7,0547 0,3178 CTl Diameter G1 7,0258 0,2177 0,0016 G2 5,8668 0,2284 ETTh Diameter G1 6,7925 0,2171 <.0001 G2 4,664 0,2277 ETTl Diameter G1 7,0017 0,1865 <.0001 G2 4,8308 0,1956 TTh Diameter G1 6,1358 0,298 0,5053 G2 5,8427 0,3125 TTl Diameter G1 7,0017 0,3615 0,6456 G2 6,7568 0,3792 Distance from the Prosthesis G2 20,1483 0,9669
CT – Trachea in its cervical portion. ETT – Trachea at the entrance to the thorax. TT- Trachea in its thoracic portion. h – height value obtained for each specific segment. l – length value obtained for each specific segment.
DISCUSSION
Mortality was 33.33% for G1 and 50.0% for G2; however, there was no statistical difference between the groups. These data, compared to those observed in other species, such as dogs undergoing extraluminal prosthesis placement, are slightly higher. This result, however, can be attributed to the fact that the present study prioritized the placement of a new intrathoracic prosthesis, whereas in other studies, the prosthesis was introduced only in the cervical region or up to the entrance of the thorax. These procedures generally have lower complication rates and consequently lower mortality (9-26%), as complications are more frequent in procedures that reach the thoracic trachea (9,12-14).
Considering the study conducted by Suematsu et al., the mortality rate was 24%. These authors implanted a spiral prosthesis up to the second rib in dogs with CT, which may justify the lower mortality rate, as they did not reach the carina as in the present study. The SHAM group, as well as the operated group, demonstrated evolution in dexterity to perform the appropriate operative technique.
According to Chisnell and Pardo (43), 47% of dogs operated on for CT with extraluminal rings developed some type of postoperative complication. Tinga, Thieman, and Mankin (9) reported that 42% of the animals evaluated also presented some type of complication. In our study, 50% of the animals in G1 and 50% in G2 developed some postoperative respiratory alteration, with no statistical difference between the groups. We observed various complications including pulmonary edema, pulmonary granuloma, death, laryngeal paralysis, pneumonia, pneumothorax, and breathing noises. Some of these complications have been reported in other studies (5, 9, 12, 14).
Among the observed complications, laryngeal paralysis is frequently reported in the literature when placing an extraluminal prosthesis (4, 15). This complication was also observed in 2 animals in the present study (16.66%), one in G1 and the other in G2. This is primarily due to the surgical technique itself, as injuries to the right or left recurrent laryngeal nerves can occur during the procedure and are not necessarily secondary to the prosthesis used.
It was noted that during the digital dissection of the intrathoracic trachea in the mediastinal region, there is a risk of the surgeon injuring the recurrent laryngeal nerve, which can lead to iatrogenic laryngeal paralysis. Therefore, it is crucial that the surgeon is well-prepared and skilled to perform the surgical procedure to avoid such complications.
As depicted in Figure 6, in one of the rabbits that presented with laryngeal paralysis, the site of injury to the recurrent laryngeal nerve was identified. In this animal, a rupture of the left recurrent laryngeal nerve was observed at the entrance to the thorax in the cranial mediastinum. This complication has been previously reported by other authors in approximately 10-30% of patients operated on with extraluminal prostheses (14, 15).
According to Chisnell and Pardo (12), iatrogenic laryngeal paralysis was present in 9% (n=2) of the animals in the recent postoperative period, and two other animals developed late laryngeal paralysis, 3 months and 5 years after the surgical procedure, resulting in a total of 17% of the animals developing laryngeal paralysis at some point postoperatively.
In a more recent study by Suematsu et al. (5), the occurrence rate of laryngeal paralysis was reported to be 2%, and this complication was observed 12 days after the surgical procedure. This suggests that it could be secondary to the presence of the prosthesis, possibly due to irritation of the recurrent laryngeal nerve caused by the prosthesis, which was not observed in our study.
Furthermore, some authors also report the persistence of respiratory clinical signs such as coughing even after the placement of an extraluminal prosthesis (5). We did not observe this type of clinical sign, as we used a different species of healthy animals. However, respiratory clinical signs secondary to complications were observed in the immediate postoperative period, such as respiratory sounds.
Respiratory sounds included wheezing, snoring, and audible stridor without the need for lung auscultation. However, these sounds were observed in animals that also presented laryngeal paralysis, pneumonia, or pulmonary edema, which are complications that can secondarily affect the bronchial tract, as seen in this study. This can be attributed to increased inflammation of the respiratory tract due to conditions such as bronchitis, tracheal collapse, and other chronic processes (16).
Considering the groups, 16.66% of animals in G1 (n=1) and 33.33% in G2 (n=2) exhibited respiratory sounds. Examining these animals individually, the one in G1 that had respiratory sounds also experienced pneumothorax in the immediate postoperative period, which was resolved by thoracentesis, developed laryngeal paralysis, and died due to respiratory complications secondary to the paralysis. In G2, one animal also developed laryngeal paralysis and succumbed to respiratory changes, while another developed pulmonary granuloma and pulmonary edema but did not die from these complications. Although G2 had more animals with complications compared to G1, there was no statistical difference between the groups.
Regarding the occurrence of pulmonary edema, only one animal in G2 (16.66%) developed this complication. This same animal also exhibited respiratory sounds and had pulmonary granuloma noted during necropsy evaluation, but did not die from these complications.
All animals underwent tomographic examination immediately postoperatively and again 14 days after surgery, while they were still alive. This analysis aimed to identify postoperative respiratory complications and evaluate the placement of the tracheal prosthesis. From the tomography, pneumothorax was identified in 25% of the animals immediately postoperatively, with 33.33% in G1 and 16.66% in G2. Upon identification, all animals underwent thoracentesis, preventing any fatalities due to pneumothorax.
The occurrence of pneumothorax is not typically reported in other studies, likely due to the use of prostheses placed only up to the thoracic entrance, as reported by Suematsu et al. (5).
However, correcting cases of intrathoracic collapse is crucial, as patients with this condition experience a lower quality of life. Currently, these patients are typically managed with intraluminal prostheses (3, 17). It is important to note that despite the lack of statistical significance, the presence of observed complications serves as a warning to surgeons to take all necessary precautions to prevent them.
The operative time in G2, where animals were implanted, varied between 16 and 24 minutes, which differs significantly from what has been reported by other authors such as Suematsu et al. (5), where operative times ranged from 120 to 150 minutes. This considerable difference can be justified by the type of prosthesis used and the surgeon’s experience, as evidenced by the clear evolution in implantation efficiency from the first to the last animal. Another noteworthy factor is that our study utilized rabbits, whereas the aforementioned work used dogs, highlighting clear anatomical differences. When considering only the prosthesis placement itself, the procedure time ranged from 5 to 7 minutes, demonstrating the feasibility of performing the technique and implanting the intrathoracic extraluminal prosthesis, provided it is done carefully.
Unlike the findings of Suematsu et al. (5), complications related to tracheal vascularization, such as vascular injury and tracheal necrosis secondary to ischemia, were not observed in rabbits. However, in agreement with these authors, Chisnell and Pardo (12) emphasized that such complications can arise due to tracheal devascularization during the dissection necessary for helical prosthesis implantation, potentially affecting at least one of the pedicles responsible for tracheal blood supply.
Conversely, other authors have reported on similar techniques in dogs without encountering these specific complications (9, 15, 18, 19). This suggests that meticulous dissection and careful tracheal manipulation are critical for successful implantation of an intrathoracic extraluminal prosthesis.
It is important to note that in our study, extraluminal implantation extended up to the thoracic trachea near the carina in rabbits, contrasting with literature studies that limited extraluminal stent placement only up to the thoracic entrance and in different species. Therefore, it is plausible that some complications observed in our study may be more akin to those seen in animals operated on with implants reaching the thoracic trachea, albeit with intraluminal prostheses. Currently, there are no studies demonstrating extraluminal prostheses with this objective.
However, complications affecting patients treated with intraluminal prostheses often differ from those observed with extraluminal prostheses. When placing intraluminal prostheses or stents, common complications include prosthesis fractures leading to secondary respiratory issues, as well as stent displacement due to their non-sutured fixation, relying solely on contact and mild compression against the tracheal mucosa (20, 22-26).
The complication of stent displacement, a concern in intraluminal prostheses, could potentially apply to our study with extraluminal intrathoracic prostheses, given the dissection of peritracheal tissue during implantation. Analyzing the implantation distance of prostheses in G2, as determined from tomographic examinations, it was observed that immediately postoperatively, prostheses were implanted at depths ranging from 15.7 to 21.6mm, while before euthanasia, depths ranged from 18.5 to 24.9mm. This analysis indicates some degree of prosthesis displacement, despite fixation with sutures at both the cranial and distal ends. However, this did not show statistically significant variation.
Furthermore, considering the total anesthesia time in our study, it ranged from 24 to 74 minutes, with an average of 43.8 minutes in G1 and 48.83 minutes in G2. This contrasts with observations by Chisnell and Pardo (12), where total anesthesia time ranged from 65 to 180 minutes, averaging 120 minutes. The discrepancy is primarily due to the different design of the extraluminal prostheses used in their study, which required more extensive suturing for fixation, thus necessitating more time for the procedure. Additionally, it’s important to note that their study did not involve intrathoracic prostheses. In our study, despite implanting the prosthesis within the thoracic cavity of the animals, total anesthesia time and consequently surgery time were shorter.
Considering the size variables of the tracheal lumen, determined by length and height measurements in various segments of the trachea, it can be inferred that the means observed for all parameters tended to be lower in G2. This is likely attributable to the presence of the prosthesis, which supports the tracheal structure through extraluminal contact. However, due to the lack of comparable studies focusing on tracheal diameter variations, direct comparisons with our results are not feasible.
Nevertheless, when analyzing tracheal segments separately, statistical differences between the groups were noted for measurements obtained in the cervical trachea and at the thoracic entrance, but not for the intrathoracic trachea. This difference may be influenced by greater mobility in each region, as the intrathoracic trachea tends to experience less movement compared to the cervical trachea and thoracic entrance.
It is important to highlight that the intrathoracic trachea adapts to external pressure conditions, unlike its more cranial portions, given the negative pressure even within the mediastinal space. This adaptation may explain the lesser degree of compression observed in the intrathoracic trachea compared to other analyzed portions.
Therefore, it can be inferred that the presence of extraluminal prosthesis, similar to other prostheses on the market, aims not to cure tracheal collapse but to improve quality of life. These prostheses provide structural support to the trachea, and while they may induce a reduction in tracheal lumen, this reduction is beneficial as it prevents dynamic collapse. This support reduces tracheal movement and consequently mitigates the inflammatory process responsible for clinical signs in patients.
Lastly, the absence of non-autologous material in contact with the tracheal mucosa, as seen with intraluminal stents, appears to lead to a lower inflammatory response and subsequently reduces the intensity and frequency of clinical signs in patients. This underscores the potential superiority of extraluminal implants for enhancing patient quality of life. However, further studies involving larger animal cohorts and longer follow-up periods are necessary to validate these findings, as the limited number of animals in our study may have impacted the observed complication rates compared to other studies.
Conclusions
When analyzing the prosthesis placement time in G2, we observed an average of 5.667 minutes with variability between 5-7 minutes, demonstrating the feasibility of implantation. The meticulous placement inside the chest, aimed at avoiding injuries, did not prove to be time-consuming. Thus, the detailed manipulation required in rabbits did not hinder the technique’s applicability and suggests its suitability for canine species.
Moreover, the satisfactory operative times support a better postoperative period with reduced anesthesia duration. This also indicates the technique’s ease and repeatability, highlighting its clinical applicability. Therefore, further studies and implants in the most affected animal species by tracheal collapse (CT), such as dogs, are warranted.
With a larger number of animals, fewer complications might be expected, as initial complications were predominantly observed in the first operated animals, suggesting a learning curve effect for the surgical team.
It is notable that most complications arose from blind implantation of the prosthesis near the animals’ carina, unlike studies using extraluminal prostheses up to the height of the first or second ribs.
However, our research did not find other similar studies with intrathoracic extraluminal implants, underscoring the necessity for additional research to better understand and manage intrathoracic CT. Patients with intrathoracic CT generally have lower quality of life compared to those with cervical CT.
Although displacement of the tracheal prosthesis was not significant in our study, it remains a potential complication. Enhanced fixation with additional suture points may mitigate this risk.
Lastly, accurate identification of the patient’s carina region is crucial for surgeons, given that many CT cases involve this area or areas caudal to it. Hence, thorough anatomical knowledge and precise intraoperative palpation skills are essential.
References
1.Lopez-Minguez S, Serrano-Casorran C, Guirola JA, Rodriguez-Zapater S, Bonastre C, De Gregorio MA. New tracheal stainless steel stent pilot study: twelve month follow-up in a rabbit model. PeerJ. 2019;7:e7797. PubMed PMID: 31608174. Pubmed Central PMCID: PMC6788445. Epub 2019/10/15.
2.Rozanski E. Canine Chronic Bronchitis. Veterinary Clinics of North America: Small Animal Practice. 2014;44(1):107-16.
3.Chick Weisse AB, Nathaniel Violette, Renee McDougall, Ken Lamb. Short-, intermediate-, and long-term results for endoluminal stent placement in dogs with tracheal collapse. Journal of the American Veterinary Medical Association. 2019;254(3):380-92.
4.Della Maggiore A. An Update on Tracheal and Airway Collapse in Dogs. Veterinary Clinics of North America: Small Animal Practice. 2020;50(2):419-30.
5.Suematsu M, Suematsu H, Minamoto T, Machida N, Hirao D, Fujiki M. Long‐term outcomes of 54 dogs with tracheal collapse treated with a continuous extraluminal tracheal prosthesis. Veterinary Surgery. 2019.
6.Tawfik A, Ebada HA, El-Fattah AMA, Kamal E. Surgical management of suprastomal tracheal collapse in children. International Journal of Pediatric Otorhinolaryngology. 2019 2019/03/01/;118:188-91.
7.Adamama-Moraitou KK, Pardali D, Athanasiou LV, Prassinos NN, Kritsepi M, Rallis TS. Conservative Management of Canine Tracheal Collapse with Stanozolol: A Double Blinded, Placebo Control Clinical Trial. International Journal of Immunopathology and Pharmacology. 2011 January 1, 2011;24(1):111-8.
8.Korman RM, White JD. Feline CKD: Current therapies – what is achievable? J Feline Med Surg. 2013 September 1, 2013;15(1 suppl):29-44.
9.Ayres SA, Holmberg DL. Surgical treatment of tracheal collapse using pliable total ring prostheses: results in one experimental and 4 clinical cases. The Canadian Veterinary Journal. 1999;40(11):787-91. PubMed PMID: PMC1540003.
10.Johnson LR, Pollard RE. Tracheal Collapse and Bronchomalacia in Dogs: 58 Cases (7/2001–1/2008). Journal of Veterinary Internal Medicine. 2010;24(2):298-305.
11.Tangner CH, Hobson HP. A Retrospective Study of 20 Surgically Managed Cases of Collapsed Trachea. Veterinary Surgery. 1982;11(4):146-9.
12.Chisnell HK, Pardo AD. Long-Term Outcome, Complications and Disease Progression in 23 Dogs After Placement of Tracheal Ring Prostheses for Treatment of Extrathoracic Tracheal Collapse. Veterinary Surgery. 2014;44(1):103-13.
13.Johnson L. Tracheal Collapse: Diagnosis and Medical and Surgical Treatment. Veterinary Clinics of North America: Small Animal Practice. 2000 11//;30(6):1253-66.
14.Tinga S, Thieman Mankin KM, Peycke LE, Cohen ND. Comparison of Outcome After Use of Extra-Luminal Rings and Intra-Luminal Stents for Treatment of Tracheal Collapse in Dogs. Veterinary Surgery. 2015;44(7):858-65.
15.Becker WM, Beal M, Stanley BJ, Hauptman JG. Survival after Surgery for Tracheal Collapse and the Effect of Intrathoracic Collapse on Survival. Veterinary Surgery. 2012;41(4):501-6.
16.Lesnikowski S, Weisse C, Berent A, Le Roux A, Tozier E. Bacterial infection before and after stent placement in dogs with tracheal collapse syndrome. Journal of Veterinary Internal Medicine. 2020.
17.Violette NP, Weisse C, Berent AC, Lamb KE. Correlations among tracheal dimensions, tracheal stent dimensions, and major complications after endoluminal stenting of tracheal collapse syndrome in dogs. Journal of Veterinary Internal Medicine. 2019;33(5):2209-16.
18.Buback JL, Boothe HW, Hobson HP. Surgical treatment of tracheal collapse in dogs: 90 cases (1983-1993). Journao of the American Veterinary Medical Association. 1996 Feb 1;208(3):380-4. PubMed PMID: 8575969. Epub 1996/02/01. eng.
19.Kirby BM, Bjorling DE, Rankin JHG, Phernetton TM. The Effects of Surgical Isolation and Application of Polypropylene Spiral Prostheses on Tracheal Blood Flow. Veterinary Surgery. 1991;20(1):49-54.
20.Durant AM, Sura P, Rohrbach B, Bohling MW. Use of Nitinol Stents for End-Stage Tracheal Collapse in Dogs. Veterinary Surgery. 2012;41(7):807-17.
21.Navas-Blanco J, Uduman J, Diaz-Mendoza J. Emergent airway management in a patient with in situ tracheal stent: A lesson learned. Saudi Journal of Anaesthesia. 2018 October 1, 2018;12(4):626-8.
22.Sun F, Usón J, Ezquerra J, Crisóstomo V, Luis L, Maynar M. Endotracheal stenting therapy in dogs with tracheal collapse. The Veterinary Journal. 2008 2//;175(2):186-93.
23.Sura PA, Krahwinkel DJ. Self-expanding nitinol stents for the treatment of tracheal collapse in dogs: 12 cases (2001–2004). Journal of the American Veterinary Medical Association. 2008;232(2):228-36.
24.Beal MW. Tracheal Stent Placement for the Emergency Management of Tracheal Collapse in Dogs. Topics in Companion Animal Medicine. 2013 8//;28(3):106-11.
25.Beranek J, Jaresova H, Rytz U. Use of nitinol self-expandable stents in 26 dogs with tracheal collapse. Schweizer Archiv fur Tierheilkunde. 2014 Feb;156(2):91-8. PubMed PMID: 24463323. Epub 2014/01/28. Eng.
26.Rosenheck S, Davis G, Sammarco CD, Bastian R. Effect of Variations in Stent Placement on Outcome of Endoluminal Stenting for Canine Tracheal Collapse. Journal Of The American Animal Hospital Association. 2017;53(3):150-8.
1. PhD student in Animal Science of North Fluminense State University Darcy Ribeiro, Campos dos Goytacazes – RJ, Brazil
2. Master’s student in Animal Science of North Fluminense State University Darcy Ribeiro, Campos dos Goytacazes – RJ, Brazil
3. Professor of Surgery of North Fluminense State University Darcy Ribeiro, Campos dos Goytacazes – RJ, Brazil
* Corresponding author – 2000, Alberto Lamego Avenue (Darcy Ribeiro North Fluminense State University); andrevet@uenf.com.br
