REGISTRO DOI: 10.5281/zenodo.8083423
Sandro Pinheiro da Costa¹
Lucas Evangelista Alves Feijão²
Lucas Amaral Cunha³
Irlane Silva Veras4
Deyvisson Luís Malta de Melo5
Nathalia Luiza Figueirôa de Carvalho6
Judit Callañaupa Yepez7
Gabriel Santos Costa da Cruz8
Rodrigo Daniel Zanoni9
Resumo
De acordo com as autoridades reguladoras, os cosméticos devem ser seguros para a saúde humana e devem ter uma avaliação de segurança, incluindo a consideração do perfil toxicológico de todos os ingredientes. Os batons são preparações cosméticas aplicadas repetidamente e diretamente nas membranas mucosas labiais. Assim, os metais em batons constituem uma fonte de exposição crônica e podem causar efeitos adversos à saúde. O objetivo geral deste estudo foi apresentar uma correlação entre os metais pesados presentes em cosméticos para uso na mucosa labial e os riscos à saúde apresentados pela exposição a essas substâncias. Trata-se de uma revisão de literatura realizada entre os meses de março de 2023 a junho de 2023. Os resultados descritos neste estudo mostram que os cosméticos labiais, por serem produtos para uso oral, podemos considerar que a ingestão do produto é quase certa. A presença de elementos metálicos tóxicos, e apesar da legislação existente no órgão de saúde brasileiro que trata de cosméticos, também é necessária uma ferramenta legislativa que considere os elementos metálicos em cosméticos para garantir a preservação da saúde pública. É possível concluir que o conhecimento de possíveis contaminantes em produtos labiais e a toxicidade dessas substâncias são ferramentas importantes para avaliar a segurança desses produtos cosméticos, o uso contínuo desses cosméticos contaminados pode resultar no aumento de alguns teores de metais no corpo humano além dos limites aceitáveis. Essas descobertas exigem programas de testes regulares obrigatórios para verificar a concentração de metais em produtos cosméticos.
Palavras-chave: Metais pesados; Contaminantes.
Abstract
According to regulatory authorities, cosmetics must be safe for human health and must have a safety assessment, including consideration of the toxicological profile of all ingredients. Lipsticks are cosmetic preparations applied repeatedly and directly to the labial mucous membranes. Thus, metals in lipsticks constitute a source of chronic exposure and can cause adverse health effects. The general objective of this study was to present a correlation between heavy metals present in cosmetics for use on the labial mucosa and the health risks presented by exposure to these substances. This is a literature review carried out between the months of March 2023 and June 2023. The results described in this study show that lip cosmetics, as they are products for oral use, we can consider that the ingestion of the product is almost certain. The presence of toxic metallic elements, and despite existing legislation from the Brazilian health agency that deals with cosmetics, it is also necessary to have a legislative tool that considers metallic elements in cosmetics to ensure the preservation of public health. It is possible to conclude that the knowledge of possible contaminants in lip products and the toxicity of these substances are important tools to evaluate the safety of these cosmetic products, continuous use of these contaminated cosmetics can result in an increase in some levels of metals in the human body beyond acceptable limits.These findings call for mandatory regular testing programs to verify the concentration of metals in cosmetic products.
Keywords: Lipsticks; Heavy metals; Contaminants.
1. Introduction
A cosmetic product is considered any substance or pharmaceutical preparation placed in contact with one of the external parts of the human body (epidermis, capillary system, nails, lips and external genitalia) or that can be applied to the teeth and/or mucous membranes of the oral cavity, and may also be used exclusively or mainly for the purpose of cleaning, perfumery, protection, change of appearance, correction of body odors and maintenance of surfaces in good condition (OYEDEJI et al., 2011). Cosmetics can be mixtures of some surfactants, oils, actives and other ingredients that must have properties and the ability to guarantee efficacy, durability, stability and be safe for human use (RAO & PRATHIBA, 1998).
Brazil is a cosmetic market with great potential for the world and several factors contribute to this. These include: the source of active ingredients and raw materials, especially those of natural origin; the use of advanced and innovative technologies; advances in the regulatory area and the search for qualification.
Many cosmetics contain chemical additives to improve their performance properties and effectiveness making them more viable products. However, there has been growing concern about the addition of chemical compounds, including dioxane, formaldehyde, lead, parabens, phthalate, aluminum, arsenic, cadmium, chromium, manganese, mercury, titanium. Although these chemicals are associated with harmful effects on human health at high levels of exposure, the levels found in cosmetics, when used properly, should be below toxic concentrations.
According to regulatory authorities, cosmetics must be safe for human health under normal or reasonably foreseeable use, and for each finished product, there must be a safety assessment, including consideration of the toxicological profile of all ingredients. et al., 2016 NASIRUDEEN & AMAECHI, 2015). Despite this, some cases of adverse health effects have been reported, including more frequently allergic reactions resulting from the presence of various chemical compounds in cosmetics. Around 10,000 chemical substances can be detected in cosmetic products, and there are more than 1,000 substances that, due to their toxicological profile, cannot be used in these cosmetic products, among which several heavy metals stand out (BOCCA, PINO & ALIMONTI, FORTE, 2014; BOROWSKA & BRZÓSKA 2015; ULLAH et al., 2013).
Lipsticks are cosmetic preparations applied repeatedly and directly to the labial mucous membranes. Thus, metals in lipsticks constitute a source of chronic exposure and can cause adverse health effects. Furthermore, in the case of metal allergens, it can occur even after a short period of aesthetic use and even after the first use in the case of sensitized individuals (BOCCA, PINO & ALIMONTI, FORTE, 2014; BOROWSKA & BRZÓSKA 2015; GOH, NG & KWOK, 1989; OH et al; 2016; TRAVASSOS et al., 2011; VERHULST et al., 2014; ZAKARIA, 2015).
For these reasons, in recent years there has been a growing interest in lipsticks as a source of toxic metal exposure and unfavorable side reactions. Cosmetics are one of the products most frequently causing allergic reactions (BOCCA, PINO & ALIMONTI, FORTE, 2014; BOROWSKA & BRZÓSKA 2015; GOH, NG & KWOK, 1989; OH et al; 2016; ACKAH et al., 2015; FARRAG & ABU, 2015, GAO et al., 2015; IBRAHIM et al., 2016; IWEGBUE et al., 2016; IWEGBUE, 2015; KALICANIN & VELIMIROVIC, 2016; NOUIOUI et al., 2016; BASKETTER et al., 2003, SMITH, CLARK & WILKINSON, 2015), however, little attention has been given to metals in these preparations as causes of allergic processes. Thus, this present study provides an overview of lipsticks as a source of metal exposure.
Thus, the general objective of this work is to present a correlation between the heavy metals present in cosmetics for use on the labial mucosa and the health risks presented by exposure to these substances.
2. Development
2.1 Methodology
This literature review was carried out between March 2023 and June 2023, with restrictions on the date of publication of the included works, papers from the last 15 years on the subject were accepted. Studies in Portuguese and English were accepted. Ongoing studies were not analyzed, being analyzed complete case-control studies, clinical trial, epidemiological and reviews on the subject available in the following databases: MEDLINE, SCIELO, SCIENCE DIRECT and VHL. Exclusion criteria were: a) articles that do not deal with the association of contaminants in cosmetics; b) articles that did not address the use of makeup on the labial mucosa; c) case reports; d) review articles that did not present data correlating heavy metals and cosmetics; e) other types of study than those listed above. An analysis of the references of each work was performed to include additional studies.
The search strategy included the terms [batom/lipstick, contaminates/contaminants, cosmetics/cosmetics, metals/metals, toxicity/toxicity] all appearing in the title and/or abstract. Corresponding terms in English were also checked. The pre-selection of studies was based on reading their title and/or abstract, and, when necessary, the full text. A search was also carried out on the current legislation for cosmetics.
2.2. Lip products
According to Law No. 6,360 of 1976, in Art. 3, V, provides that cosmetics are products that were manufactured for external use, and their purpose designates the protection and beautification of the various parts of the body. The following are considered as beautification products for external application: lipstick, lip pencils, blushes, make-up bases, facial powders, cosmetic oils, mascaras, eye shadows, eyeliners, hair dyes, hair bleaching agents, talcum powders, among others (BRASIL, 1976).
Lipstick is a cosmetic product that contains pigments, oils, waxes and emollients that apply color, which are adjusted to the desired melting point and viscosity, give texture and protection to the lips, as with most other types of makeup, lipstick it is typically, but not exclusively, used by women. Some lipsticks also have a protective purpose, to add color and hydration. The product consists of a stick-shaped material, inside a tubular container, usually around 10 mm in diameter and 50 mm in length (BALSAM & SAGARIN, 2008). According to Balsam and Sagarin (2008), lipsticks must have the following characteristics:
a) It should be smooth and easy to apply and leave a thin film on the lips;
b) It must be non-irritating and non-toxic;
c) Must have slow wear, moisture, color and shine.
d) It should be free of grit and provide hydration and plasticity.
e) It must be innocuous both internally and externally.
The main components present in lipstick formulations are described in Table 1.
Table 1: Lipstick components and functions.
Component Function Solid Waxes (beeswax, carnauba wax, candelilla wax) provide hardness softening agents Lubricates lipstick after application (wool fat, lanolin, lecithin, cocoa butter) Dispense the pigment and give the lipstick a high gloss Oil (castor oil, liquid paraffin) color coloring agents give aroma Perfumes Formulation stabilization
Source: The author (2023).
The proportion of ingredients used in lipstick formulations determines the final characteristics of the product. In general, they are made from an oily vehicle comprising fat or oil hardened to a desired consistency with waxes of various types which also serve to raise the melting point and improve physical stability. The color is normally provided by insoluble pigments, such as some intensely colored metals, generally used finely dispersed in the oily vehicle, and one or more different dyes may be associated in the formulation, many of these ingredients are described in Table 1 (BATISTA, 2017).
With all this it is important to point out that with constant growth and the integration of new proposals and different brands, beauty products, mainly lipstick, gain a margin of development proportional to its stimulation with its final consumer, through beauty advertisements among others.
According to Batista (2017), there is an increasing number of people using makeup on a daily basis, with different aspects, some for aesthetic reasons, others for socialist reasons, others for formalities, work, and others because they like it. to enhance the look, for this reason they acquire these types of products with a certain frequency. Associated with this growing use of lipstick, we can consider that there is an increase in the offer of these products, including the emergence of different brands that present different options for coloring and textures, which can lead to the commercialization of products that do not meet the quality standards and can generate user health risks.
2.3. Contaminants in lip products
Most of the information needed to assess the potential risk of a cosmetic product results from knowledge of the ingredients that make up its formula. They are the ones who can be directly responsible for any local and systemic effect. However, the formulation of the product can also interfere, as it facilitates the total or partial absorption of the ingredients. In addition, possible interactions may occur, resulting from the association between the components of the formulation, which may influence the potential risk of a product (BRASIL, 2012).
According to Bocca et al. (2014), between the years 1989 and 2008, articles on metals in cosmetics were rarely published, as can be seen in Figure 1. On the other hand, between 2009 and 2013, the number of published works increased significantly from of the year 2011. The categories of cosmetic products and the respective publications can be seen in Figure 1 (BOCCA et al., 2014).
Figure 1. Articles published over the years and the respective cosmetic product category (Adapted from BOCCA et al., 2014).
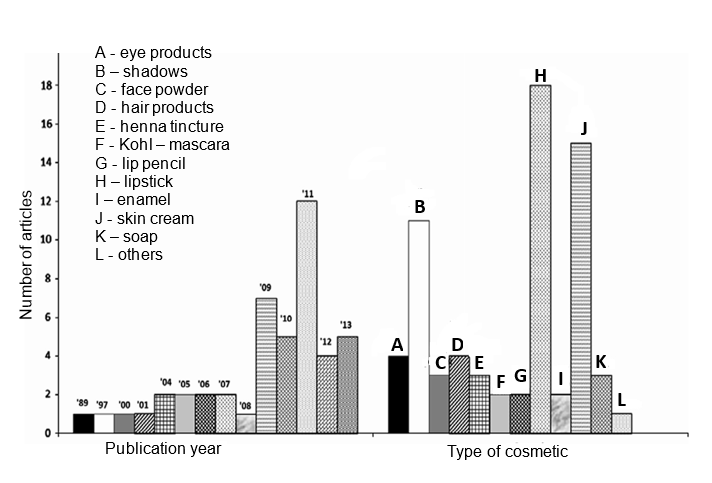
The Brazilian Association of Personal Hygiene, Perfumery and Cosmetic Industry (ABIHPEC) indicates that Brazil is among one of the biggest consumers of products with cosmetic purpose, some of the main related products are perfumes, deodorants, sun protection products and products men, occupying the fifth ranking as the largest makeup product market, a considerable placement given the vast field of personal hygiene and cosmetics industries that the country has (ABIHPEC, 2016).
Constant growth and the integration of new proposals to serve the consumer and the emergence of different brands of beauty products, mainly lipstick. Due to this growth, the risk of the presence of contaminants in these products is increasing.
The sources of exposure and/or contamination by heavy metals in cosmetic products can be divided into two main categories. Firstly, there are the sources of metals destined for intentional use in the production of cosmetics, which include pigments, UV filters and preservatives, as well as antiperspirant, antibacterial and antifungal agents. Occurring mainly exposure to metals such as chromium, iron, aluminum, zinc, titanium, strontium, copper, silver and gold. The type of metal will vary according to its application in the formulation and the exposure according to the concentration, which should vary according to the regulatory laws of each country. The other important category of exposure/contamination to metals in cosmetics is unintentional source impurities resulting from the use of raw materials (plants and minerals) and water contaminated with metals, as well as the use of metal-coated apparatus during production of cosmetics (FDA, 2017).
During the various stages of cosmetics production, undesirable constituents that can easily be present as sources of contamination include lead, cadmium, aluminum, mercury and arsenic. It is important to highlight that the same metal can be present in a cosmetic product due to its intentional use and also present itself as an impurity (BOCCA et al., 2014). Table 2 presents different metals intentionally used in the production of cosmetic products.
Table 2: List of metals intentionally used in the production of cosmetics.
Metal Goal Product Reference Chromium, Titanium, Iron, Silver, Copper, Cobalt, Bismuth hair coloring Paints and Shampoos KANG et al., 2006 Chromium oxide and chromium hydroxide dyes eye shadows KANG et al., 2006 Chrome Dye Lipstick ZAKARIA & HO, 2015 Iron oxide Dye eye makeup KANG & LEE, 2006; ZAZARIA & HO, 2015 Zinc, copper, iron and chromium Durability Cosmetics in general BOCCA et al., 2014 Titanium dioxide, zinc oxide and barium sulfate UV filters sunscreens FAGE et al., 2016 Mercury Preservatives and skin lighteners Makeup and creams ADAWE & OBERG, 2013 Aluminum Dyes, antiperspirants and astringents Makeup, aftershave, astringent lotions, mouthwash, deodorants, bath salts MARINOVICH et al., 2014
Source: The author (2023).
The different inorganic metallic compounds are used in the production of cosmetics, many of them as dyes (chromium, titanium, iron), hair dyes and shampoos (silver, copper, iron, cobalt, bismuth). Substances such as chromium oxide, chromium hydroxide and iron oxide are used as dyes in the preparation of cosmetics applied to the eyelids of the eyes (eye shadows), some other essential metals (zinc, copper, iron and chromium) can also be added to cosmetics during production in order to improve pigment quality; however, if they are present in excessive amounts, they can be a cause of skin irritation and other adverse effects (ATZ, 2008).
Nanoparticles of titanium dioxide, zinc oxide and barium sulfate are used as physical ultraviolet filters in skin care and protection products (KANG et al., 2006). Mercury compounds are used as preservatives in eye makeup products. Due to the ability of mercury to inactivate tyrosinase (the main melanin-forming enzyme), in some countries mercury is used in skin lightening creams (ADAWE & OBERG, 2013; MARINOVICH et al., 2014). Aluminum, in addition to its use as a dye, is also used in antiperspirants, as it is able to block sweat glands. Furthermore, due to the astringent properties (precipitation of blood proteins) allowing the inhibition of microvascular bleeding, aluminum compounds are used in aftershave preparations, other astringent facial lotions, bath salts, and mouthwashes. ; BOCCA et al., 2014; FAGE et al., 2016)..
2.4. Aluminum (Al) and aluminum oxide (Al2O3)
Aluminum is a chemical element with the symbol Al, being considered a metallic element, it has atomic number 13 and its atomic mass 27 u (KROSCHWITZ, 1999).
Alumina, also known as aluminum oxide (Al2O3), is its dehydrated form. This compound is related to the production of colors which come from small impurities of heavy metals. The most common natural form of alumina is its crystalline form. This water-insoluble inorganic solid can form various other crystalline phases and also an amorphous form. Each phase has a unique crystalline structure and varies in chemical properties, such as its acid-base reaction rate. Aluminum hydroxide, also known as hydrated alumina, is most commonly found as a polymorphic mineral (a component of the aluminum ore known as bauxite) (KROSCHWITZ, 1999; BALAN et al., 2013).
Aluminum oxide also known as alumina is reported to function in cosmetics as an abrasive, absorbent, anti-caking agent, bulking agent and opacifying agent; aluminum hydroxide is reported to function as a buffering agent, corrosion inhibitor and pH adjuster (SLANIN et al., 1984).
Aluminum in the form of aluminum hydroxide is poorly absorbed orally or by inhalation and is essentially not absorbed dermally in healthy humans. Usually orally, approximately 0.1% to 0.6% of aluminum is absorbed. It is believed that the main mechanism of absorption occurs in the gastrointestinal tract system through passive diffusion of paracellular pathways. Absorbed aluminum is distributed to all organs, but mainly in bone and lung tissues, its excretion occurs in the urine and, to a lesser extent, in the bile (SLANINA et al, 1984, DOMINGO, GOMEZ & LLOBE, 1991, TESTOLIN, et al., 1996).
Dermal absorption can occur through the aluminum salts used in antiperspirants (manufacture of aerosols) that form a precipitate of denatured keratin hydroxide in the cornified layer that surrounds and obstructs the opening of the sweat ducts, these mechanisms suggest that there is little or no no dermal absorption of aluminum hydroxide or any other form of aluminum (YOKEL & MCNAMARA, 2001; PRIEST et al., 1996, WEBERG & BERSTAD, 1986; THORNE et al., 1987).
2.5. Arsenic (As)
Arsenic is a chemical element with the symbol As, being considered a metal, with atomic number 33 and atomic mass 75 u (EFSA, 2009).
Arsenic found as a commonly distributed element in the atmosphere, rocks, minerals, soil, water and the biosphere. Redox potential and pH are the most important factors controlling arsenic speciation. Toxicity varies with the chemical form in which it is found, and can range from essentially non-toxic to excessively toxic, depending on the following factors: oxidation state and groups attached to arsenic. Arsenic hydride, known as arsine, is considered to be the most toxic species, while arsene sugars (AsSug, found in algae) and arsenobetaine (AsB, found in fish) are considered non-toxic (EFSA, 2009).
Arsenic toxicity may be linked to its ability to block the SH group of different enzymes. The uncoupling of phosphorylation and the breakdown of essential metabolic processes, such as glucose metabolism, lead to the denaturation of different proteins (CHEN, 2009).
In cosmetics segments incorporating reddish pigments, for example, are commonly contaminated with arsenic. Some cosmetics that are colored and used for face, body, hair care and herbal cosmetics may contain relatively high amounts of this heavy metal in their formulation. This element, like other heavy metals, can accumulate in the skin and internal organs, causing toxic effects, which can be classified as topical effects (mainly contact dermatitis) or systemic effects (systemic allergic dermatitis). In addition to causing dermatological disorders, they can generate circulatory and peripheral nervous system imbalances, increase the risk of lung cancer, a possible increase in the risk of cancer of the gastrointestinal tract and urinary system (BOCCA et al., 2014; BOROWSKA & BRZOSKA, 2015). Therefore, through the simple daily use of a cosmetic applied to the labial mucosa, such as lipsticks and gloss, for example, this metal can accumulate and consequently cause toxic and harmful effects to the health of the consumer.
2.6. Cadmium (Cd)
Cadmium is a chemical element with the symbol Cd, being considered a transition metal, it has atomic number 48 and its atomic mass consists of 112.4 u, at room temperature it tends to remain in its solid state (UCKO, 1992).
According to Bocca et al. (2014), cadmium is heavily used by the cosmetics industry as a pigmenting agent, used in lipstick shadows and in the most varied cosmetics that require coloring. Cd sulfide has a yellow color and little by little if a favorable amount of the element selenium is added, it can have a color variant between shades of orange to darker tones, reaching shades close to black. The addition of cadmium (yellow) with Cr(III) oxide results in a light green tonality pigmentation, known by cadmium green industries (BOCCA et al., 2014).
After ingestion, cadmium in the body acts directly on cell proliferation, differentiation and apoptosis. These activities interact with the DNA repair mechanism, the generation of reactive oxygen species (ROS) and the induction of apoptosis. Cadmium binds to mitochondria and can inhibit cellular respiration and oxidative phosphorylation at low concentrations. This mechanism results in chromosome disturbances, DNA strand breaks, and cross-linking between DNA and protein. Cadmium can induce ROS production and result in oxidative stress. This mechanism may express the role of cadmium in organ toxicity, carcinogenicity and apoptotic cell death (RANI et al., 2015; PATRICK, 2003; JOSEPH, 2009).
2.7. Lead (Pb)
Lead is a chemical element with the symbol Pd, being considered a heavy metal, it has atomic number 82 and its atomic mass consists of 207.2 u (CDC, 1991).
Lead (Pb) is an element that occurs naturally in the earth’s crust. It is widely distributed throughout the environment as it has been used in gasoline production, consumer products, product recycling, and manufacturing processes (CDC, 1991). Although important measures have been implemented in several countries to decrease environmental exposure to Pb, such as the use of unleaded gasoline, removal of lead from paint, soldering of canned foods and glazed ceramics used for food storage and preparation, it is still a major environmental health problem in specific communities and high-risk target populations (MEYER et al., 2008).
The ingestion of large amounts of lead causes intoxication by interfering with the synthesis of the heme group at the mitochondrial level and in calcium channels in nerve conduction. Lead affects virtually every system in the body, including the reproductive, neurological, hematopoietic, hepatic, and renal systems. It is well established that lead can cross the placenta during pregnancy and has been associated with intrauterine fetal death and premature births (PAPANIKOLAOU et al., 2005; COMISKEY et al., 2015; MEYER et al., 2008).
The concentration of approximately 10 lg/dl of lead in maternal blood is enough to increase the risk of hypertension in pregnancy and miscarriage. In addition, the consequences of accelerated bone loss during menopause and the decrease in estrogen production can be increased due to high levels of lead in the body (VAHTER et al., 2007; BELLINGER, 2005).
In the cosmetic industry, inorganic Pb can be found in hair dyes (such as lead acetate) and lipsticks and can be minimally absorbed by the skin, unlike organic lead, which is quickly absorbed. Studies show that dermal absorption of inorganic lead occurs at a rate of 2.9 ng/cm. Exposure to organic and inorganic compounds lead to significant contamination of lead on the skin, which may originate from cosmetics for cutaneous use (ALSAFFAR & HUSSEIN, 2014; NNOROM, IGWE & OJI-NNOROM, 2005; HARDY, WALTON & VAISHNAV, 2004).
However, lead exposure assessments have always been based on ingestion of food, water or air. Lead is generally not an ingredient in lipstick, but it can be found as an impurity in raw materials or acquired during the manufacturing process. Most recent studies emphasize that there is no safe level of exposure to lead, representing a much smaller source of exposure compared to other sources (food and water, for example), due to the amount of lipstick that is applied daily. However, it cannot be excluded that lead accumulates in the body over time and repeated application of lipsticks containing lead can lead to significant levels of exposure. The consequences of these products can only be adequately verified by conducting an exposure study to assess population risk (BELLIRGER, 2008, VOLPE et al., 2012; COMISKEY et al., 2015).
2.8. Chrome (Cr)
Chromium is considered as a transition metal and heavy metal, its atomic number is 24, its atomic mass consists of 52 u, and at a temperature of around 21 and 25°C it is stable assuming its solid characteristic (UCKO, 1992).
The next most stable form is hexavalent chromium. Cr (0), Cr (III) and Cr (VI) are used commercially and are present in the environment. Cr(0) is primarily in its metallic form as a component of iron-based alloys such as stainless steel. Trivalent chromium (Cr(III)) is mainly of geological origin and hexavalent chromium (Cr(VI)) is mainly derived from industrial processes but can also originate from the oxidation of natural Cr(III) by minerals. ; UCKO, 1992).
The main source of oral exposure to Cr for non-occupational human populations comes from ingestion of food and drinking water. Increased industrial applications, however, lead to a large amount of Cr released into soil, groundwater and air. The specific mechanism of chromium carcinogenicity remains unclear, however, there are data supporting the genotoxicity and mutagenicity of Cr(VI) in vivo and in vitro. Chromium in its hexavalent form is considered a procarcinogen. Cr(VI) manages to enter the cell through the mechanism of molecular mimicry as a polyatomic ion followed by its metabolic reduction to Cr(V), Cr(IV) and to the trivalent form in the final reduction (SALNIKOW & ZHITKOVICH 2008; ALEXANDER & AASETH 1995; O’BRIEN et al. 2001).
Occupational exposure usually occurs by inhalation and the lung is the main target organ, and there are also reports that exposure can occur through the skin. The widespread incidence of dermatitis noted among construction workers is attributed to their exposure to chromium present in cement, as in a study presented by Shelnutt et al., 2007.
In general, occupational and environmental exposure to compounds containing Cr (VI) is known to cause toxicity in multiple organs, such as kidney damage, allergy and asthma, and cancer of the respiratory tract in humans (COSTA, 1997). Chromium toxicity occurs through oxidation state and solubility. Cr(VI) compounds, which are powerful oxidizing agents and therefore tend to be irritating and corrosive, may be more systemically toxic than Cr(III) compounds due to similar amount and solubility (CONNETT & WETTERHAHN, 1983 ; DE FLORA et al., 1990)
The biological interaction mechanisms are still uncertain, and may be related to the ease with which Cr (VI) can pass through cell membranes and its subsequent intracellular reduction to reactive intermediates. As Cr(III) is poorly absorbed, toxicity is mainly attributed to its Cr(VI) form. The main organs related to the absorption of this metal are the lungs and the gastrointestinal tract (DAYAN & PAINE, 2001).
2.9. Mercury (Hg)
Mercury is a chemical element with the symbol Hg, considered as a metal, its atomic number is 80, its atomic mass consists of 200.59 u (CAITO et al., 2018).
Mercury exists in both organic and inorganic forms. The inorganic form can be further subdivided into elemental mercury and mercury salts. Elementary exposures are typically from mercury-containing devices, such as thermometers, for example. Exposures to mercury salt can be observed with the ingestion of batteries or medicinal compounds with the presence of mercury in their formulation, such as laxatives. Organic mercury can also be subdivided into short-chain and long-chain compounds. Exposure to organic mercury usually occurs through eating contaminated seafood, hair dyes or even other skin contact cosmetics (CAITO et al., 2018; BJORKLUND, 2017).
Long-term exposure to mercury becomes harmful to health, this exposure when it reaches the systemic circulation, the transport of this substance will occur through organic anions in the kidneys occurs through the kidney of the proximal tubules (HAZELHOFF, BULACIO & TORRES, 2012 ; HULTMAN & ENESTROM, 1986).
2.10. Manganese (Mn)
Manganese is a chemical element with the symbol Mn, considered as a transition metal, its atomic number is 25, its atomic mass consists of 55 u (GUNIER et al. 2013).
Manganese is an essential metal that plays a key role in brain development and functioning, it is also widely used in the processing of other metals. It is an essential trace element, found in a wide variety of food sources, including meat, fish, poultry, dried fruit, tea and nuts. Environmental exposure to Mn can lead to accumulation in the basal ganglia and the development of Parkinson-like disorders (BORGESE et al. 2013; GUNIER et al. 2013).
Manganese is a naturally occurring trace element essential for human development and brain function and other biological processes. As a trace element, Mn is taken up with the diet (mainly with grains, fruits, vegetables, tea) and, once ingested, is absorbed by the small intestine in a proportion of about 3-4%. Gastrointestinal absorption is influenced by iron metabolism: an iron deficiency increases Mn absorption through some transport proteins. When exposure occurs by inhalation, Mn is absorbed through the alveolar-capillary membrane in a percentage between 40 and 70%, its elimination is through the gastrointestinal tract through bile, intestinal mucosa and pancreatic secretion. The main excretion of Mn is carried out in the feces regardless of the route of introduction, while the portion excreted in the urine is low, about 6% of the total (DEWITT et al. 2013; LUCAS et al. 2015).
2.11. Titanium (Ti)
Titanium (Ti) is a non-essential metallic element with atomic number 22, being an insoluble compound. It is the ninth most abundant element in the Earth’s crust and the seventh most abundant metal overall (BARKSDALE, 1968).
In powder form, Ti is one of the whitest substances available, making it an ingredient in a wide range of industrial and consumer products, including cosmetics and sunscreens. Notably, titanium is the third most used material in consumer products. The use of Ti in sunscreens makes it a more transparent vehicle, less viscous and adheres to the skin more easily (ROBICHAUD et al., 2009; HUSSAIN et al., 2012; NEWMAN, STOTLAND & ELLIS, 2009)
The widespread topical use of Ti occurs through nanoparticles which increase its use in cosmetic products, having the potential to penetrate through the stratum corneum, reach the viable layers of the skin and ultimately result in sensitization or other side effects. The permeability of the oral mucosa becomes up to 4000 times greater than that of other tissues, which would facilitate the penetration of cosmetic products applied to the oral mucosa, making exposure to this heavy metal even more intense. Exposure to Ti in personal care products is common, but reports of clinical allergy are rare. However, evidence for possible toxic effects and risk of allergic reactions is weak.
2.12. Toxicological aspects: health risks
There are several regulatory agencies that are dedicated to the control and regulation of commercial activities, safety and quality control of cosmetics. Although there are rules and quality control tests to be followed for the manufacture of a cosmetic, these regulatory mechanisms are not fully effective, as adverse effects still persist in the population of cosmetic consumers (ALANI, DAVIS & YIANNIAS, 2013).
Faced with the emergence of the use of cosmetic products and greater exposure to the compounds in formulas for a long time and frequency, the side effects of these products become more frequent in the population. Many components present in the composition of cosmetics are also pollutants to the environment, these contaminations occurring mainly through water, representing health risks (NICOLOPOULOU-STAMATI, HENS & SASCO, 2015).
Thus, Cosmetovigilance aims to ensure the safety of cosmetic products for commercial purposes. This vigilance is very important to control potentially dangerous ingredients. Unlike drugs, there is no specific agency to assess the safety of cosmetic products, no marketing authorization with specific requirements, no assessment of the risk-benefit ratio and no guarantee of constancy from one batch to another (VIGAN & CASTELAIN, 2014) .
The health risks associated with the use of cosmetic products are currently an emerging public health problem, where about 12% of users in the general population have had undesirable effects with one or more cosmetic products in the last nine years (NICOLOPOULOU-STAMATI , HENS & SASCO, 2015; VIGAN & CASTELAIN, 2014).
The presence of components in the formulation of cosmetic products, such as heavy metals, has the potential to cause side effects and its consequences can vary from a simple mild hypersensitivity reaction to an anaphylactic process or even lethal intoxication, since there is a series of adverse reactions caused by cosmetics. Most adverse reactions are irritating, however, type IV hypersensitivity, contact urticaria, photosensitization, pigmentary disorders, hair and nail damage, paronychia, acneiform eruptions, folliculitis, and exacerbation of an established dermatosis may also occur. The body areas most affected by adverse reactions attributed to the use of cosmetics are the head, lips and neck, and irritant dermatitis is the most common type of complication (DRAELOS, 2015; ALANI, DAVIS & YIANNIAS 2013; PARK & ZIPPIN, 2014; WOLF et al., 2001).
Metals present in cosmetics can be accumulated in the skin or absorbed through this route, metals such as Ni, Co and Cr are accumulated in the stratum corneum and can cause allergic contact dermatitis, while Hg, Pb, Cd and Al pass through the layers of the skin into the bloodstream and are transported to various organs where they accumulate and exert toxic effects (STAUBER et al., 1994; LANSDOWN & SAMPSON, 1996; LIN et al., 2012, LARESE et al., 2007; FILON et al., 2009 ).
Although metals are present in cosmetics in very low concentrations, repeated use of these products can result in significant cumulative exposure to metals, sometimes numerous. It is worth noting that cosmetics containing metals represent a source of occupational exposure for hairdressers and beauticians, for example, due to their frequency in handling these products (FOULDS, 2006; HOSTYNEK, 2003).
Several exposure scenarios are possible for metals present in cosmetics, which can result in brief contact, as with rinse-off products (e.g., shampoos, shower gels, toothpastes) or remaining in contact with the skin for long hours, as in products leave-on products (e.g., face creams, body lotions, lipsticks), where these preparations can be applied to a large body surface area (FOULDS, 2006; HOSTYNEK, 2003).
Increased absorption can occur when cosmetics come into contact with areas such as the genital region, as well as the skin of children, in whom absorption through the skin is greater than in adults. In the case of metals present in lipsticks, there is a risk of oral ingestion, while the ingredients of products applied in the form of sprays can be absorbed by inhalation, exposure to the metal depends on numerous exogenous and endogenous factors that determine the permeability of the skin to them. (FOULDS, 2006; HOSTYNEK, 2003).
Metals such as nickel, chromium, cobalt and selenium accumulate mainly in the stratum corneum and can cause skin effects (eg allergic contact dermatitis).
Nickel compounds are generally soluble and diffusible, being able to penetrate the stratum corneum through skin appendages (hair follicles, sweat glands and sebaceous glands), as well as by transcellular or intracellular routes, but the penetration of this metal into the stratum corneum is slow and addresses only 1% of the amount applied to the skin. However, it should be taken into account that absorption can be altered by factors that influence skin permeability (BOCCA et al., 2014; HOSTYNEK, 2003).
At the epidermal level, the metal, being a hapten (non-protein substance, low molecular weight, alone cannot induce an immune response), binds to amino acid residues of proteins, forming metal-protein complexes capable of causing contact allergy. Allergic reactions are the most common adverse skin effect of metals in cosmetics, and nickel is the most important metal allergen in these products. Reported cases of allergy due to metals present in cosmetics were mainly caused by colored cosmetics containing nickel, cobalt and chromium (BOCCA et al., 2014; BOROWSKA & BRZÓSKA, 2015; SMITH, CLARK & WILKINSON, 2015; GOH & KWOK, 1989; OH et al., 2016; TRAVASSOS et al., 2011).
The metals mercury, cadmium, lead, aluminum and nickel are recognized for their ability to be systemically absorbed and transported to different organs, which can accumulate and cause damage to internal organs, resulting in neurological disorders; cardiovascular problems; liver, kidney and lung damage; reproductive and developmental disorders and cancers (CHAN et al., 2001; BENZ et al., 2011).
From these toxicological considerations, it is important to highlight that the legislative document structures that regulate cosmetics are essential to ensure safe levels of contaminants in cosmetics, in order to ensure the integrity of the health of users.
2.13. Regulatory aspects
Documentary structures that regulate cosmetics differ significantly between countries and are far from harmonized. In general, cosmetic products are subject to regulatory controls in all countries, with the aim of ensuring the safety of using the products and avoiding adverse effects on the health of consumers. It is important to highlight that the lack of harmonization between markets can lead to customs barriers (RATH & CANAES, 2009).
2.13.1. Brazilian legislation for cosmetic products
The “Guide for the Safety Assessment of Cosmetic Products”, which was first published in 2003, describes that the safety assessment of cosmetic products must precede the placing of the cosmetic product on the market. The company is responsible for the safety of the cosmetic product, as guaranteed by the Term of Responsibility presented at the time of product regularization, where the company declares that it has supporting data that attest to its effectiveness and safety. Since the cosmetic product is freely accessible to the consumer, it must be safe under normal or reasonably foreseeable conditions of use. The pursuit of this security must permanently incorporate the advancement of the state of the art in cosmetic science (BRASIL, 2012a).
2.13.2. RDC nº 211 of July 14, 2005
According to RDC nº 211, of July 14, 2005, it establishes the Definition and Classification of Personal Hygiene Products, Cosmetics and Perfumes. Personal Hygiene Products, Cosmetics and Perfumes, are preparations made from natural or synthetic substances, for external use in the various parts of the human body, skin, capillary system, nails, lips, external genital organs, teeth and mucous membranes of the cavity oral, with the sole purpose of cleaning, perfuming, changing the appearance or correcting body odors, and protecting, keeping it in good condition. There are different classifications for these products which are:
- Grade 1 products: are personal hygiene products, cosmetics and perfumes, which, due to their basic or elementary properties, whose proof is not initially necessary and do not require detailed information regarding their mode of use and their respective restrictions, due to the characteristics its intrinsic properties, such as, for example, some cosmetics mentioned in the list of grade 1 products, deals with products such as lip balm and lip gloss (not for photoprotective purpose), facial concealer (not for protective purpose), eyeliner for lips, eyes and eyebrows, among others. others.
- Grade 2 products: are personal hygiene products, cosmetics and perfumes, whose formulations have specific indications and evidence, as well as their mode of use, information regarding care and restrictions. For example, some cosmetics mentioned in the grade 2 list, including: children’s lipstick and children’s lip gloss, children’s blush/rogue, children’s cologne, anti-dandruff/hair loss conditioners and children’s conditioners, deodorant for intimate use, among others.
It is possible to observe that there are different classifications regarding personal hygiene products, grade 1 products do not require detailed information when compared to grade 2 products, which can provide products with the presence of contaminants in their formulation, presenting a risk to health of the final consumer, since these products are for cutaneous use, which allows considerable permeation, which may cause harmful effects, such as the presence of heavy metals commonly correlated mainly to grade 1 products, due to approval by the inspection body being less rigid, therefore, it is expected that these products do not present an imminent risk to the health of the buyer.
2.13.3. RDC Nº 48, October 25, 2013
Approves the Technical Regulation of Good Manufacturing Practices for Personal Hygiene Products, Cosmetics and Perfumes, and takes other measures. RDC Nº 48/13 through its quality management the principles to be followed from the beginning of the production of cosmetics, personal hygiene and perfume to the commercialization process. Through the context discussed, follow the Basic Requirements of Good Manufacturing Practices:
“Manufacturing processes must be clearly defined, systematically reviewed, and show that they are capable of manufacturing products within the required quality standards, meeting the respective specifications”
“Critical steps in the manufacturing processes and any significant modifications must be systematically controlled and, where possible, validated”
“The manufacturing areas must be provided with the necessary infrastructure to carry out the activities, including:
I. Trained and qualified personnel;
II. Adequate facilities and spaces;
III. Appropriate services and equipment;
IV. Appropriate labels, packaging and materials;
V. Approved instructions and procedures;
VI. Appropriate deposits;
VII. Adequate personnel, laboratory and equipment for quality control.”
This RDC aims at quality management, from the first stage of cosmetic manufacturing, its critical stages and after its validation, with this it seeks to portray the possible achievements of the appropriate activities such as training, checking of services and materials and the necessary clothing equipment, where they influence the quality of the final product, in which a safe and effective product will be presented to the consumer.
2.13.4. RDC Nº 47, March 16, 2006
Approves the Technical Regulation List of Allowed Ultraviolet Filters for Personal Hygiene Products, Cosmetics and Perfumes. The Resolution provides for the prevention of ultraviolet filters, and the substances that are incorporated into sun protection products, where they are intended to filter ultraviolet rays, with the aim of protecting the skin from the harmful effects caused by the same.
As an example, the Methyl Sulfate of N, N, N-trimethyl – 4- (2, oxoborn – 3 – ylidenemethyl) anilinium Camphor benzalkonium methosulfate, which presents 6% as the maximum concentration authorized by current Brazilian legislation.
The search for prevention with ultraviolet filters, has been breaking several paradigms regarding the improvements of their filters and percentage that are determined for the filters to provide protection, and that many of these formulations at the time of their preparation do not exceed the pre-established limit.
2.13.5. RDC Nº 29, June 1, 2012
Approves the Mercosur Technical Regulation on the “List of Substances with Preservative Action Allowed for Personal Hygiene Products, Cosmetics and Perfumes” and makes other provisions. All personal hygiene products, cosmetics and perfumes must be guaranteed through normal or foreseeable conditions of use. It is essential to periodically update the lists of substances in order to guarantee the correct use of raw materials in the manufacture of personal hygiene products, cosmetics and perfumes.
According to RDC No. 29, of June 1, 2012, which lists the preservative substances allowed for personal hygiene products, cosmetics and perfumes. One of the mentioned preservatives is the metal Titanium, presented in other formulas as Silver chloride, deposited in titanium dioxide, having its maximum concentration authorized by legislation 0.004% (calculated as silver chloride). It has its limitations, such as 20% AgCI (w/w) in TiO2. This preservative is restricted for children under 3 years of age, and in products for oral hygiene and in products for the eyes and lips. This makes it difficult to manufacture some isolated cosmetics, considering that this same preservative, Titanium, cannot be in cosmetics that are directly in contact with the mucosa and has its limitations regarding the minimum age.
2.13.6. RDC Nº 48, March 16, 2006.
Provides for the approval of the technical regulation on the list of substances that cannot be used in personal hygiene products, cosmetics and perfumes.
It is considered one of the functions of the Sanitary Surveillance, to maintain as a basis the prevention of health problems, the population the regulatory action to guarantee the quality of products and services, which includes the approval of norms and their respective updates, as well as the due inspections of their application, thus being considered as personal hygiene products, cosmetics and perfumes, they must remain safe under normal or foreseeable conditions for their users.
The use in cosmetic products of substances in categories I and II of the IARC (which is the international agency for research on cancer), as well as other international references, which are also classified as carcinogenic, mutagenic or toxic for reproduction in the general. Continuing the list, some possible metals are pointed out, but the current legislation does not indicate their percentages of use, it only prohibits them, as is the case of heavy metals Arsenic, Lead, cadmium, Mercury and its compounds.
With this, it is valid to point out the fragility of some Brazilian legislation as well as the same one addressed, since the same metals that are cited and addressed in this resolution can be pointed out in other lists, remaining free of exceptions.
2.14. Comparison between Brazilian and international legislation for cosmetic products
The European Union legislation cited in (Annex IV of the EU Directive) allows the use of oxide green and hydroxide of Cr (III) as dyes in formulations in the cosmetic field as is normally found, and these dyes may have Cr (VI) which are not allowed by legislation in the same country, on the other hand it may lead to divergences regarding (Annex II) (EU, 1976, 2009) where it says that the products that were marketed with this substance cannot go to the sales circuit (DIAS & RAU, 2013; BOCCA et al., 2014).
Table 3: Comparison between the limit of contaminants allowed for lipsticks in Brazil, USA and European Union
Substances Brazil (ANVISA) USA (FDA) European Union arsenic Prohibited Variable limits according to the dye Prohibited Lead (up to 3ppm as an impurity in dyes) Variable limits according to the dye Prohibited Cadmium Prohibited There is no set limit Prohibited Chrome (up to 20ppm as an impurity in dyes) Permitted in the form of dye Prohibited Aluminum Prohibited Permitted in the form of dye Permitted in the form of dye Manganese Prohibited Permitted in the form of dye Permitted in the form of dye Titanium Permitted in the form of dye Permitted in the form of dye Permitted in the form of dye Mercury Permitted in the form of dye Variable limits according to the dye Prohibited Phthalate Permitted in the form of dye There is no set limit Prohibited
Source: DIAS & RAU, 2013
2.15. Discussion
The presence of metallic substances in lip products has become an issue of concern, therefore, through a quantification study. Liu et al. (2013) measured the concentration of lead and eight other metals in samples of lip products and assessed the potential health risks related to the estimated consumption of these metals. Most of the lip products tested contained high concentrations of titanium and aluminum. All products examined had detectable manganese. Lead was detected in 24 products (75%), with an average concentration of 0.36 ± 0.39 ppm, including one sample with 1.32 ppm.
The authors concluded that the safety of cosmetics should be evaluated not only by the presence of substances dangerous to health, but by the frequency of use and consequent exposure to the contaminating substance. Although metal concentrations in lip products have been reported across studies around the world, the interpretation of how reported concentrations may be related to potential health risk is still a challenge (Liu et al., 2013).
The presence of toxic metals in lip cosmetics was addressed by Maehata et al. (2015). This study evaluated three brands of short-lasting commercial lipsticks, considering red and pink, and considering low, medium and high cost. The authors noticed the difference in the amount of metallic element both between the colors (red and pink) and between the price ranges, that is, there is no guarantee that the price will determine greater or lesser quality in the product, considering that the lipstick of high cost showed a higher amount of nickel.
Marta et al. (2017) conducted a study to determine the presence of metals in lipsticks sold in Brazil. This study looked at different lipstick brands. The data obtained in the study can be seen in Table 4.
Table 4: Element concentration ranges determined in lipstick samples.
Element Concentrations found in the samples (µg.g-1) As 0,001 until 0,080 Pb 0,010 until 0,2 Cd <0,01 until 0,03 Cr <0,01 until 2,5 Hg <0,01 until 0,2 Al <0,01 until 4.500 Mn <0,1 until 40 Ti 0,9 until 100
Fonte: Adaptado de MARTA et al., 2017).
According to Marta et al. (2017), in European legislation, all elements studied are prohibited, except aluminum, which is allowed in dye, whereas in American legislation, all are allowed in the form of dye with variable limits. The authors indicate that these results demonstrate that certain contaminants are present in lipstick samples, although prohibited by Brazilian legislation, which can cause various diseases, harming the population that uses them.
In a study carried out by Batista, Augusto and Pereira-Filho (2016) the presence of lead in cosmetic samples was observed. Lead was previously detected in 25 lipstick samples, lead concentrations ranged from 0.11 to 4.48 mg/kg. While in a study by Al-Saleh et al. (2009) reported that four brands of lipstick exceeded the FDA pre-established lead limit as impurities (20 ppm). The United States FDA has approved the use of mica (silicate minerals that provide a shiny, sparkling appearance) in good manufacturing practices with a lead content not exceeding 20 ppm in drugs, dentifrices, and externally used cosmetics (USFDA, 2002).
These heavy metals should not be added to cosmetics during the manufacturing process as an ingredient formula. However, it is possible to observe the presence of these substances in lipsticks in different studies. The existence of heavy metals was believed to be due to the natural occurrences of these heavy metals in color additives, as well as contamination in the lipstick manufacturing process. During the manufacturing process, sources of heavy metals can come from lead and solder paints in manufacturing equipment and also from lead-contaminated dust from the manufacturing environment.
To ensure safety and effectiveness, cosmetic products are regulated and controlled around the world. However, the harmonization of laws dealing with cosmetics is far from being achieved and regulatory frameworks vary greatly between countries, making it practically impossible for a global industry to sell the same product in all markets (AMPARO & ALBERTO, 2007)
They can contain over 10,000 ingredients related to many diseases such as cancer, birth defects, developmental and reproductive harm. Knowing such toxic effects, FDA (2011), completely banned the presence of nine ingredients, including coal tar colors, formaldehyde, glycol ethers, lead, mercury, phenylenediamine, phthalates in cosmetic products.
Exposure to metals can occur through diet, medications, environmental exposure and use of cosmetics (ADAL & TARABAR, 2013). For example, underarm antiperspirant use was investigated by Darbre (2003) as a possible cause of breast cancer. The basis for breast carcinogenesis may be due to the binding of various chemical constituents including metals to DNA and promoting the growth of damaged cells, which is why some guidelines prohibit the use or presence of heavy metals such as: Cd, Co, Cr, Ni and Pb considered impurities in the preparation of cosmetics.
The Agency for Toxic Substances and Disease Registry (2012) stated that exposure to more than 0.005 ppm of cadmium is likely to be hazardous to human health. However, the exposure level that is well thought of as a high limit is diverse because the cadmium effect can increase or decrease depending on other factors such as form, type and duration of exposure..
Although metal concentrations in cosmetic products have been reported by studies conducted in many countries, the interpretation of how these reported concentrations may be related to potential health risk is a challenge (ADEPOJU-BELLO et al., 2012. AL-SALEH, AL-ENAZI & SHINWARI, 2009; BRANDÃO et al., 2012). The increase in health awareness and the increased availability of cosmetic products in different markets calls the attention of researchers in order to find adverse effects related to contamination by heavy metals (SAEED et al., 2010; BARAKAT, 2011).
Therefore, lip cosmetics, as they are products for oral use, we can consider that the ingestion of the product is almost certain. The results described above in this chapter showed the presence of toxic metallic elements, and, although there are current legislations of the Brazilian health agency that deals with cosmetics, it is also necessary a legislative tool that considers metallic elements in cosmetics to ensure the preservation of the public health. In order to fully evaluate the safety of cosmetic products containing metals, post-marketing surveillance and monitoring are necessary, mainly through Cosmetovigilance, which aims at a set of activities related to the detection, evaluation, understanding and prevention of adverse events and any other problems. associated with cosmetic products.
An example of the application of Cosmetovigilance is the Sanitary Surveillance Center of São Paulo (CVS/SP), which makes available to the population and health professionals an electronic notification form that facilitates communication by users about problems arising from the use of cosmetics. , such as technical complaints or undesirable effects. Then, based on the analysis of the notifications received, it is possible to monitor the effects resulting from the use of cosmetics, personal care products or perfumes that are available on the market, as well as monitoring compliance with current legislation (prohibition of substances, labeling, among others). ) (BRASIL, 2018).
Resolution RDC nº 332 of 1st. of December 2005, determines that responsible manufacturing/importing companies in Mercosur must implement a Cosmetovigilance system. This system should cover the registration of reports of occurrences of adverse events/evaluation, the registration of the adopted measures and the notification to ANVISA. Through the Sanitary Surveillance Notification System (Notivisa), registered companies/institutions, health professionals and the citizen (by means of a citizen form) can make notifications of situations that imply a risk to the health of the consumer, thus, according to the ANVISA, regulatory actions may be applied such as modification of labeling information, publication of alerts and guidelines for rational use, review of ingredients used and their concentrations for use in cosmetics, up to the withdrawal of batches from the market, suspension of the product and cancellation of the record.
Therefore, verification of compliance with specifications should be seen as a necessary requirement to guarantee the quality, safety and efficacy of the product, and not just as a regulatory requirement (BRASIL, 2008).
3. Conclusion
The work presented addresses a currently discussed topic, where every day several studies are carried out and published through electronic articles, journals and books. Knowing the possible contaminants in lip products and the toxicity of these substances are important tools to assess the safety of these cosmetic products and for them to be used safely without exposure to substances that are harmful to health. In addition, this review study made it possible to survey the currently available legislation, continuous use of these contaminated cosmetics can result in an increase in some levels of metals in the human body beyond acceptable limits. These findings call for mandatory regular testing programs to verify the concentration of metals in cosmetic products in order to limit their increase in the body and thus protect the health of consumers. Additional efforts are needed to educate users and the general public about the dangers of using these products outside the specifications outlined by regulatory agencies.
4. References
ABABNEH, F.A., ABU-SBEIH, K.A., AL-MOMANI, I.F. Evaluation of allergenic metals and other trace elements in personal care products, Jordan Journal of Chemistry, 8(3): 179 – 190, 2013
ABIHPEC. Panorama do Setor de HPPC. Associação Brasileira da Indústria de Higiene Pessoal, Perfumaria e Cosméticos, p. 1–22, 2016.
ADAL A, TARABAR A. Heavy Metal Toxicity. 2013.
ADEPOJU-BELLO AA, OGUNTIBEJU OO, ADEBISI RA, OKPALA N, COKER HAB. Evaluation of the concentration of toxic metals in cosmetic products in Nigeria. Afr J Biotechnol.; 11: 16360 – 16364. 2012
ADEPOJU-BELLO, A. A., OGUNTIBEJU, O. O., ADEBISI, R. A., OKPALA, N.,
ALANI JI, DAVIS MDP, YIANNIAS JA. Allergy to cosmetics: A literature review. Dermatitis. 24(6):283–90, 2013
ALEXANDER J, AASETH J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. Analyst. 120:931–933, 1995
ALSAFFAR NM, HUSSEIN HJ. Determination of heavy metals in some cosmetics available in locally markets. J Environ Sci Toxicol Food Technol, 8(8): 09 e12., 2014
AL-SALEH I, AL-ENAZI S, SHINWARI N. Assessment of lead in cosmetic products. Regul Toxicol Pharmacol. 54: 105-113. 2009
AL-SALEH, I.; AL-ENAZI, S.; SHENWARI, N. Assesment of lead in cosmetic products. Regul. Toxicol. Pharmacol. 54, 105-113. 2009
AMAPARO S & ALBERTO C: General Concepts and Cosmetic Legislation, In: Analysis of cosmetic products. Elsevier; Kidlington, Oxford. 1 – 42. 2007
AMASA, W., SANTIAGO, D., MEKONEN, S., AMBELEU, A. Are cosmetics used in developing countries safe? Use and dermal irritation of body care products in Jimma Town, Southwestern Ethiopia. Journal of Toxicology, 1 – 8., 2012
ANDREAE M.O., in Organometallic compounds in the environment: principles and reactions, ed. by P.J. Craig, New York, Wiley, p. 198. 1986
ANVISA. Brasil. Brasília: Agência Nacional de Vigilância Sanitária, 2012.
ATSDR: Agency for Toxic Substances and Disease Registry, (2012): Public Health Statement for Cadmium: September 2012.
ATZ, V. L. Desenvolvimento de métodos para determinação de elementos traço em sombras para áreas dos olhos e batom. Dissertação (Dissertação Química) UFRS. Porto Alegre, p. 75. 2008.
AYENIMO, J.G., YUSUF, A.M., ADEKUNLE, A.S., MAKINDE, O.W. Heavy metal exposure from personal care products, Bull Environ Contam Toxicol, 84; 8 – 14; 2010.
BALAN E, LAZZERI M, MORIN G, MAURI F. First-priciples study of the OH-streching modes of gibbsite. American Mineralogist. 91:115- 119. 2013
BALSAM M.S.; SAGARIN E. Cosmetics science and technology, Second ed. Wiley Interscience Publication, NY, USA, 3, pp. 209-512., 2008
BARAKAT MA. New trends in removing heavy metals from industrial waste water. Arab. J. Chem. 4: 361–77. 2011
BARKSDALE J. Titanium. In: The Encyclopedia of the Chemical Elements, Hampel C A (ed.): New York, Reinhold Book Corporation. pp. 732–738. 1968
BATISTA, E.F.; AUGUSTO, A.D.S.; PEREIRA-FILHO, E.R. Chemometric evaluation of Cd, Co, Cr, Cu, Ni and Pb concentration in lipstick samples intended to be used by adults and children. J. Talenta.150, 206-212. 2016
BATISTA, L. S., Determinação de chumbo em batons e tinturas capilares por espectrometria de absorção atômica. Trabalho de Conclusão de Curso. Curso de Química Licenciatura, Universidade Federal da Fronteira Sul. 44 pág., 2017.
BELLINGER, DC. Teratogen update: lead and pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 73, 409–420, 2005.
BENZ MR, LEE SH, KELLNERr R, DOHLEMANN C, BERWECK S. Hyperintense lesions in brain MRI after exposure to a mercuric chloride-containing skin whitening cream. Eur J Pediatr. 170:747–50. 2011.
BJORKLUND G, DADAR M, MUTTER J, AASETH J. The toxicology of mercury: Current research and emerging trends. Environ. Res. Nov;159:545-554. 2017
BOCCA B, PINO A, ALIMONTI A, FORTE G. Toxic metals contained in cosmetics: a status report. Regul Toxicol Pharmacol. 68:447–67, 2014
BORGESE L, FEDERICI S, Zacco A, Gianoncelli A, Rizzo L, Smith DR, Donna F, Lucchini R, Depero LE, Bontempi E. Metal fractionation in soils and assessment of environmental contamination in Vallecamonica. Italy Environ Sci Pollut Res. 20:5067–75, 2013
BOROWSKA S, BRZOSKA M.M. Metals in cosmetics: implications for human health. J Appl Toxicol. 35:551–72, 2015
BOROWSKA S, BRZOSKA M.M. Metals in cosmetics: Implications for human health. J Appl Toxicol. 35(6):551–72, 2015.
BRANDAO JDO, OKONKWO OJ, SEHKULA M, RASELEKA RM. Concentrations of lead in cosmetics commonly used in South Africa. Toxicol Environ Chem. 94: 70–77. 2012
BRASIL, 2005C. Agência Nacional de Vigilância Sanitária. Regulação – RDC Nº 211, de 14 de julho de 2005.
BRASIL, 2006e. Agência Nacional de Vigilância Sanitária. Resolução – RDC Nº 47, de 16 de março de 2006
BRASIL, 2006g. Agência Nacional de Vigilância Sanitária. Resolução – RDC Nº 48, DE 16 DE MARÇO DE 2006
BRASIL, 2008. Agência Nacional de Vigilância Sanitária (Anvisa). Guia de controle de qualidade de produtos cosméticos /Agência Nacional de Vigilância Sanitária. 2ª ed., revista. Brasília (DF): Anvisa; 2008.
BRASIL, 2012a. Agência Nacional de Vigilância Sanitária. Guia para Avaliação de Segurança de Produtos Cosméticos, 2ª edição. Brasília, DF, Anvisa, 2012.
BRASIL, 2012b. Agência Nacional de Vigilância Sanitária. Resolução – RDC Nº 44, de 9 de agosto de 2012.
BRASIL, 2012f. Agência Nacional de Vigilância Sanitária. Resolução – RDC Nº 29, DE 1 DE JUNHO DE 2012
BRASIL, 2013d. Agência Nacional de Vigilância Sanitária. Resolução – RDC Nº 48, DE 25 DE OUTUBRO DE 2013
BRASIL,2018. Cosmetovigilância. Disponível em: <http://www.cvs.saude.sp.gov.br/apresentacao.asp?te_codigo=25> Acesso: setembro de 2018.
BRASIL. Decreto n° 79.094, de 5 de janeiro de 1977. Regulamenta a Lei no 6.360, de 23 de setembro de 1976, que submete a sistema de vigilância sanitária os medicamentos, insumos farmacêuticos, drogas, correlatos, cosméticos, produtos de higiene, saneantes e outros. Diário Oficial da União, Brasília, DF, 05 jan. 1977.
CAITO SW, JACKSON BP, PUNSHON T, SCRIMALE T, GRIER A, GILL SR, LOVE TM, WATSON GE, VAN WIJNGAARDEN E, RAND MD. Editor’s Highlight: Variation in Methylmercury Metabolism and Elimination Status in Humans Following Fish Consumption. Toxicol. Sci. Feb 01;161(2):443-453.2018
CDC, Center for Disease Control and Prevention. Preventing Lead Poisoning in Young Children: A Statement by the Center for Disease Control. US Department of Health and Human Service/Public Health Service/Center for Disease Control, Atlanta, 1991
CHAN MHM, CHEUNG RCK, CHAN IHS, LAM CWK. An unusual case of mercury intoxication. Br J Dermatol. 144:192–4. 2001
COKER, H. A. B. Evaluation of the concentration of toxic metals in cosmetic products in Nigeria, Afri. J. Biotechnol, 11(97): 16360 – 16364. 2012.
COMISKEY D, API AM, BARRATT C, DALY EJ, ELLIS G, MCNAMARA C, O’MAHONY C, et al. Novel database for exposure to fragrance ingredients in cosmetics and personal care products. Regul Toxicol Pharmacol; 72:660e72, 2015.
COMISKEY D, API AM, BARRATT C, DALY EJ, ELLIS G, MCNAMARA C, O’MAHONY C, et al. Novel database for exposure to fragrance ingredients in cosmetics and personal care products. Regul Toxicol Pharmacol; 72:660e72, 2015.
CONNETT PH, WETTERHAHN KE. Metabolism of carcinogenic chromate by cellular constituents. Struct Bonding. 54:93–24, 1983.
COSTA M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Critical Reviews in Toxicology. 27:431–442, 1997.
DARBRE PD. Underarm cosmetics and breast cancer. J Appl Toxicol. 23: 89-95. 2003
DAYAN AD, PAINE AJ. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol. 20(9):439–451, 2001
DE FLORA S, BAGNBASCO M, SERRA D, ZANACCHI P. Genotoxicity of chromium compounds: a review. Mutat Res. 238:99–172, 1990
DEWITT MR, CHEN P, ASCHNER M. Manganese efflux in Parkinsonism: insights from newly characterized SLC30A10 mutation. Biochem Biophys Res Commun. 432:1–4, 2013
DOMINGO J.L, GOMEZ M, LLOBET J.M, CORBELLA J. Influence of some dietary constituents on aluminum absorption and retention in rats. Kindney International. 39:598-601.1991
DRAELOS ZD. Cosmetics: The Medicine of Beauty. J. Cosmet Dermatol.14 (2):91, 2015
DRAELOS, Z.D. Are cosmetics safe?, Journal of Cosmetic Dermatology, 11: 249 – 250. 2012.
EFSA. Scientific Opinion on Arsenic in Food. EFSA Panel on Contaminants in the Food Chain (CONTAM), 2009
ELBE, S. ‘Our Epidemiological Footprint: The Circulation of Avian Flu, SARS, and HIV/AIDS in theWorld Economy’,Review of International Political Economy, 15: 116–130, 2008.
EMÍDIO DIAS, A. C.; RAU, C. Contaminantes em Batom: riscos e aspectos regulatórios. Dissertação (Pós-graduação em Vigilância Sanitária) PUC. GOIÁS GO, p. 20. 2017.
FDA, Food and Drug Administration Title 21–Food and Drugs. Chapter I–Food and Drug Administration, Department of Health and Human Services. Part 80– Color Additives Certification, 2017
FDA, Food and Drug Administration. Title 21–Food and Drugs. Chapter I–Food and Drug Administration, Department of Health and Human Services. Part 70– Color Additives, 2017
FDA, Food and Drug Administration. Title 21–Food and Drugs. Chapter I–Food and Drug Administration, Department of Health and Human Services. Part 74– Listing of Color Additives Subject to Certification, 2017
FERREIRA DA ROCHA, A. ”Cádimo,Chumbo,Mercurio- A problemática destes metáis pasados na saúde Publica? Monografia (Monografia de Ciência da Nutrição) FCNAUP. Porto, Portugal PT, p. 70. 2008/2009.
FILON FL, D’AGOSTIN F, CROSERA M, ADAMI G, BOVENZI M, MAINA G. In vitro absorption of metal powders through intact and damaged human skin. Toxicol. Vitro 23: 574–579.2009
Foulds IS. Facial eczema due to colour pigments in foundation makeup in nickel sensitive patients. Contact Dermatitis. 55(Suppl. 1):11; 2006
GOH CL, NG SK, KWOK SF. Allergic contact dermatites from nickel in an eye shadow. Contact Dermatitis. 20:380–1, 1989
HAMILTON, T., GANNES, G.C. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Therapy Letter, 2011.
HARDDY AD, WALTON R, VAISHNAV R. Composition of eye cosmetics (kohl) used in Cairo. Int J Environ Health Res. 14(1):83e91, 2004;
HAZELHOFF MH, BULACIO RP, TORRES AM. Gender related differences in kidney injury induced by mercury. Int. J. Mol. Sci. 13 : 10523-10536, 2012
HEPP, N.M. Determination of total lead in 400 lipsticks on the U.S. market using a validated microwave-assisted digestion, inductively coupled plasma-mass spectrometric method. J Cosmet Sci. 63 (3): 159–176, 2012.
HOSTYNEK JJ. Factors determining percutaneous metal absorption. Food Chem Toxicol. 41:327–45; 2003
HULTMAN P., ENESTROM S. Localization of mercury in the kidney during experimental acute tubular necrosis studied by the cytochemical Silver Amplification method. Br. J. Exp. Pathol. 67:493–503, 1986.
HUSSAIN S, SMULDERS S, DE VOOGHT V et al. Nano-titanium dioxide modulates the dermal sensitization potency of DNCB. Part Fibre Toxicol 2012: 9: 15, 2012.
IBRAHIM S. Y, FAWZI, M. M, SAAD M.G, RAHMAN S.M.A. Determination of heavy metals and other toxic ingredients in henna (Lawsonia inermis). J Environ Anal Toxicol; 6:3.2016.
JOSEPH P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol. 2009;238:272–9.
KROSCHWITZ J. Kirk-Othmer Concise Encyclopedia of Chemical Technology. 4 ed. New York: John Wiley & Sons, Inc, 1999.
LANSDOWN ABG, SAMPSON B. Dermal toxicity and percutaneous absorption of cadmium in rats and mice. Lab. Anim. Sci. 46: 549–554, 1996
LARESE F, GIANPIETRO A, VENIER M, MAINA G, RENZI N. In vitro percutaneous absorption of metal compounds. Toxicol. Lett. 170: 49–56, 2007
LIN SH, WANG XR, YU ITS, TANG WJ, LI J, LIU BY. Lead powder use for skin care and elevated blood lead level among children in a Chinese rural area. J. Expo. Sci. Environ. Epidemiol. 22: 198–203. 2012
LUCAS EL, BERTRAND P, GUAZZETTI S, DONNA F, PELI M, JURSA TP, LUCCHIN R, SMITH DR. Impact of ferromanganese alloy plants on household dust manganese levels: implications for childhood exposure. Environ Res.138:279–90, 2015
MAEHATA, P.; SEO, E.S.M.; SALVADOR, V.L.R. Presença de metais tóxicos em cosméticos labiais. In: Congresso Brasileiro de Cosmetologia, 12 – 14 de maio, 2015. São Paulo, SP.
MEYER, P.A., BROWN, M.J., FALK, H. Global approach to reducing lead exposure and poisoning. Mutat. Res. 659, 166–175, 2008;
NASIRUDEEN M.E, AMAECHI A.U. Spectrophotometric determination of heavy metals in cosmetics sourced from Kaduna metropolis. Nigeria Sci World J. 10:1–5, 2015
NEWMAN M D, STOTLAND M, ELLIS J I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 61: 685–692, 2009
NICOLOPOULOU-STAMATI P, HENS L, SASCO AJ. Cosmetics as endocrine disruptors: are they a health risk? Rev Endocr Metab Disord. 16(4):373–83, 2015
NNOROM IC, IGWE JC, OJI-NNOROM CG. Trace metal contents of facial (make-up) cosmetics commonly used in Nigeria. Afr J Biotechnol. 4 (10):1133 e 8, 2004
O’BRIEN T, XU J, PATIERNO SR. Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol Cell Biochem. 222:173–182, 2001
OH JE, LEE HJ, CHOI YW, BYUN JY. Metal allergy in eyelid dermatitis and the evaluation of metal contentes in eye shadows. JEADV. 2016;30:1518–21, 2016
OYEDEJI FO, HASSAN GO, ADELEKE BB. Hydroquinone and heavy metal levels in cosmetics marketed in Nigeria. Trends Appl. Sci. Res. 6:622-639, 2011.
PAPANIKOLAOU, N.C., HATZIDAKI, E.G., BELIVANIS, S., TZANAKAKIS, G.N.,
PARK M.E., ZIPPIN J. H. Allergic contact dermatitis to cosmetics. Dermatol Clin [Internet]. 32(1):1–11, 2014
PATRICK L. Toxic metals and antioxidants: Part II The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003;8:106–28
PRIEST N.D., TALBOT J.G., DAY J.P., KING S.J., FIFIELD K., CRESSWELL R.G. The bioavailability of 26Al-labelled aluminum citrate and aluminum hydroxide in volunteers. BioMetals. 9:221-228. 1996.
RAMOS M.; COSTA M. Os efeitos do chumbo sobre o organismo humano e seu significado para a saúde., Pag 129, 2004.
RANI A, KUMAR A, LAL A, PANT M. Cellular mechanisms of cadmium- induced toxicity: a review. Int J Environ Health Res. 2014;24:378–99
RAO N, PRATHIBA S. Cosmetics and personal care products. Elsevier Inc pp. 380-382, 1998;
RATH, S., CANAES, L. S. Contaminação de produtos de higiene e cosméticos por n-nitrosaminas. Quim. Nova, Vol. 32, No. 8, 2159-2168, 2009.
ROBICHAUD C O, UYAR A E, DARBY M R et al. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol: 43: 4227–4233, 2009
SAEED M, MUHAMMAD N, KHAN H, KHAN SA. Analysis of toxic heavy metals in branded Pakistani herbal products. J. Chem. Soc. Pak. 32: 471–475. 2010
SALNILOW K, ZHITKOVICH A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 21:28–44, 2008
SHELNUTT SR, GOAD P, BELSITO DV. Dermatological toxicity of hexavalent chromium. Crit. Rev Toxicol. 7:375–387, 2007.
SLANINA P, FALKEBORN Y, FRECH W, CEDERGRE A. Aluminium concentrations in the brain and bone of rats fed citric acid, aluminium citrate or aluminum hydroxide. Food and Chemical Toxicology. 22(5):391-397. 1984
SMITH VM, CLARK SM, WILKINSON M. Allergic contact dermatitis in children: trends in allergens, 10 years on. A retrospective study of 500 children tested between 2005 and 2014 in one UK Centre. Contact Dermatitis. 74:37–43, 2015
STAUBER JL, FLORENCE TM, GULSON BL, DALE LS. Percutaneous absorption of inorganic lead compounds. Sci. Total Environ. 145: 55–70. 1994.
TESTOLIN G, ERBA D, CIAPPELLANO S, BERMANO G. Influence of organic acids on aluminum absorption and storage in rat tissues. Food Additives and Contaminants. 13(1):21-27. 1996
THORNE B.M., COOK A., DONOHOE T, LYON S, MEDEIROS D.M, MOUTZOUKIS C. Aluminum toxicity and behavior in the weanling Long-Evans rat. Bulletin of the Psychonomic Society. 25(2):129-132. 1987
TRAVASSOS AR, BRUZE M, DAHLIM J, GOOSSENS A. Allergic contact dermatitis caused by nickel in a green eye pencil. Contact Dermatitis. 65:307–8. 2011
TSATSAKIS, A.M. Lead toxicity update. A brief review. Med. Sci. Monit. 11, RA329–RA336, 2005
ULLAH H, NOREEN S, FOZIA, REHMAN A, WASEEM A, ZUBAIR S, ADNAN M, AHMAD I. Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan. Arab J Chem. 10:10–8. 2013
US FDA. United State Food Drug and Cosmetic Act on Hazardous Chemicals in Cosmetics. 2011.
USFDA. Title 21-Food and Drugs. Chapter 1-Food and Drugs Administration, Department of Health and Human Services. 2002, Part 73.
VAHTER, M., ÅKESSON, A., LIDÉN, C., CECCATELLI, S., BERGLUND, M. Gender differences in the disposition and toxicity of metals. Environ. Res. 104, 85–95, 2007
VIGAN M, CASTELAIN F. Cosmetovigilance: definition, regulation and use “in practice.” Eur J Dermatology. 24(6):643–9, 2014.
VOLPE M, NAZZARO M, COPPOLA R, RAPUANO F, AQUINO R. Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA. Microchem J. 101:65e9, 2012.
VOLPE, M.G., NAZZARO, M., COPPOLA, R., RAPUANO, F., AQUINO, R.P. Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA, Microchemical Journal, 101: 65 – 69. 2012.
WEBERG R., BERSTAD A. Gastrointestinal absorptionof aluminum from single doses of aluminum containing antacids in man. European Journal of Clinical Investigation. 16(5):428-432. 1986.
WOLF R, WOLF D, TUZUN B, TUZUN Y. Contact dermatitis to cosmetics. Clin Dermatol. 19(4):502– 15 (2001).
YOKEL R.A.; MCNNAMARA P.J. Aluminum toxicokinetics: An updated minireview. Pharmacology & Toxicology. 88:159-167. 2001
ZHITKOVICH A. Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol. 24:1617–1629, 2011
1Centro Universitário Serra dos Órgãos (UNIFESO), Teresópolis, Rio de Janeiro, Brasil.
2Universidade Federal do Ceará (UFC), Ceará, Ceará, Brasil
3Universidade Federal de Alagoas (UFAL), Maceió, Alagoas, Brasil
4Faculdade Santa Terezinha (CEST), São Luís, Maranhão, Brasil
5Universidade de Pernambuco (UPE), Recife, Pernambuco, Brasil
6Universidade Federal de Pernambuco (UFPE), Recife, Pernambuco, Brasil
7Universidade Federal do Rio Grande (FURG), Rio Grande, Rio Grande do Sul, Brasil
8Universidade Federal do Sul da Bahia (UFSB), Itabuna, Bahia, Brasil
9Centro de Longevidade Irineu Mazutti, Sumaré, São Paulo
