REGISTRO DOI: 10.5281/zenodo.10735449
Larisse Silva Dalla Libera1
Jessica Enocencio Porto Ramos2
Igor Lopes Santos3
Vera Aparecida Saddi4
ABSTRACT
Introduction: The prevalence of anal cancer has grown considerably and human papillomavirus (HPV) plays a fundamental role in the etiology of these cancers, but in recent years a possible causal role for HPV in colorectal cancer (CRC) has also been investigated. Objective: To describe the prevalence of HPV infection and its genotypes in colorectal and anal cancer in men and women worldwide. Method: Systematic review performed on the databases, PUBMED, LILACS and SCIELO, identified until June 2020. The prevalence of HPV infection in the investigated tumors was estimated by combining data from 42 articles (5834 samples). The x2 test and odds ratio with a 95% confidence interval were used, considering p <0.05. Results: HPV was detected in 88.9% of cases of anal cancer, 10.9% of colorectal cancers, 5.5% in colorectal lesions, polyps and adenomas; 1.2% in colonic tissue adjacent to the tumor and free of neoplasia and 1% in healthy colonic tissues. Genotypes 16 and 18 were the most prevalent in both tumors. HPV was significantly associated with anal squamous cell carcinoma (p <0.0001). Conclusion: HPV may have a causal role in anal cancer, but not in colorectal cancer.
Key words: Human papillomavirus. Anal cancer. Colorectal cancer.
INTRODUCTION
The incidence of colon cancer (1,148,515 cases), rectum (732,210 cases) and anus (50,865 cases), increased considerably, with 1,931,590 new cases per year and 935,173 deaths (576,858 colon, 339,022 rectum and 19,293 anus) worldwide (1). Genetic susceptibility plays a fundamental role in a subset of CRC cases, but the vast majority of tumors are sporadic and not inherited (2,3). Thus, environmental causes such as infections by pathogens have been investigated as possible causal factors for these cancers (4,5). Anal carcinomas, although rare, are mostly associated with persistent high-risk human papillomavirus (HPV) infections, with HPV 16 and 18 being the most prevalent genotypes (6,7).
HPV is the most common sexually transmitted infection in the population, with sufficient evidence of its carcinogenic effects in different locations (8–10). In colorectal cancer the association of HPV has been investigated in colon and rectal tumors, as well as in precursor lesions and normal colonic tissues, but the results are still controversial (11–14).
Some risk factors can influence susceptibility to viral infection and consequent anal carcinogenesis, such as risky sexual behavior, men who have sex with men, co-infection with the human immunodeficiency virus (HIV), women with intraepithelial cervical lesions, history of anogenital cancer and immunosuppression (15–17). However, the route of viral infection to the colon or rectum is still speculative and includes transmission of HPV through the anal route or contamination during the analysis (18).
Thus, an update of the studies that investigated the association of HPV in colorectal cancer is necessary, since its causal role in these tumors is still questionable (11–14,19,20). In addition, the detection and genotyping of HPV in anal cancers is essential to assess its oncogenic potential and the impact of anti-HPV vaccines on the incidence of these cancers (21). Thus, this review aims to describe the prevalence of HPV infection and its genotypes in colorectal and anal cancers in men and women worldwide.
METHODS
Eligibility Criteria
This study comprises a systematic review of the literature in which complete articles in Portuguese or English were included, assessing the prevalence of HPV and its genotypes in anal carcinomas. The selected articles empoyedPCR and reverse hybridization techniques in paraffinized tissues. The following were excluded: review studies, case reports, studies that investigated only precursor lesions such as anal intraepithelial neoplasms (NIAs); studies that evaluated only a specific group of patients (only men or women, only HIV positive or men who have sex with men, etc.) and studies that used HPV detection by hybrid capture or immunohistochemistry techniques.
The prevalence of HPV in colon and rectum cancers in men and women, was performed for a period of ten years. Studies that evaluated only precursor and benign lesions of the colon and rectum, such as polyps and adenomas, were excluded.
Search and Selection Strategy
The search and collection of data were carried out by means of a search protocol, prepared by the authors, containing a topic of interest, inclusion criteria, search and selection strategies, for obtaining the collected data, analysis and presentation of results and interpretation of the results of the studies.
The publications were consulted on the MEDLINE database through PUBMED; in Latin American and Caribbean Literature in Health Sciences (LILACS) and in the Scientific Electronic Library Online (SCIELO). The consultation period was from January 2020 to June 2020.
The process of finding and selecting articles was conducted by two researchers independently and blindly to avoid selection bias. When the researchers were unable to establish whether the article would be included or not, a third researcher completed the decision-making process.
To assess the prevalence of HPV in anal and colorectal cancer, bolers and search terms MESH and DECS were used: papillomavirus OR HPV AND anal cancer AND prevalence. And papillomavirus OR HPV AND colorectal cancer AND prevalence.
In order to ensure the inclusion of all articles relevant to the topic, a manual search was carried out in the reference list of review articles, consensus and articles located with this search strategy. No contact was made with clinical researchers to verify possible ongoing research. Duplicate publications have been removed manually.
Data extraction
For each study included, the following were extracted: author; periodical; year of publication; study region; sample size; sample groups, HPV prevalence, sample size tested for HPV DNA; research year; histological type (when stated); origin of the material for DNA extraction from HPV; Primer used to detect HPV DNA; prevalence of HPV genotypes and presence of single or multiple HPV infections. The data extraction was carried out by the researchers independently, in disagreement a third reviewer helped to decide what information would be collected. The figure 1 presents the flowchart of the study search and selection strategy.

Figure 1. Flow diagram outlining the study selection process.
Quality assessment
This study was carried out according to 27 items included in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) (22) statement to report recommendations for reporting systematic review and meta-analysis studies. The selected articles were evaluated using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria (23).
Statistical Analysis
Estimates of HPV prevalence were expressed as percentages of all cases tested for HPV DNA and also when there was information by sex, age, study region, sample group and histological type of the tumor. The x2 test and odds ratio with a 95% confidence interval were used, considering p <0.05.
From anal cancer studies that presented estimates of anal intraepithelial lesions and invasive anal cancer, only invasive anal cancer data were collected and the total number of samples was also separated.
Estimates of the prevalence of HPV genotypes were expressed as percentages of all cases tested and the analyzes were limited to those studies that genotyped at least HPV 16 and 18. HPV genotypes were defined according to the included studies, such as high-risk HPV genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 and low-risk genotypes: 6, 11, 32,34, 40, 42, 43, 44, 53, 54, 55, 61, 70, 72, 73, 74, 81, 83, 84 and 89.
RESULTS
A total of 1384 abstracts were identified, but only 42 studies met the inclusion criteria (7,17,24–63) totaling 5,834 anal and colorectal samples tested for HPV detection (Figure 1).
Detection and genotyping of HPV in anal cancer
A total of 1842 cases of anal cancer were tested for HPV, and the prevalence of the virus in these tumors was 89% (95% CI 87.52-90.38), with prevalence variation between 60.6% to 100% in different studies studies (7,24,30–33,40,41,45,46,48,50,52,56–58). Table 1 presents the characteristics of the studies that investigated the prevalence of HPV in anal cancers.
All studies were retrospective cross-sectional studies and the average age of the subjects ranged between 50 and 65.5 years. More than half of the studies evaluated a period equal to or greater than ten years. The largest study was carried out with 496 cases of anal cancer, diagnosed over a period of 25 years, in 24 countries from different continents (7).
All detection methods were based on the polymerase chain reaction (PCR) technique and used paraffinized samples. The primers most used for HPV detection were GP5/6 and MY09/11 (30,32,40,46,57,58), however four studies did not mention the primer set used (31,33,50,52).
Overall, the prevalence of HPV in anal cancers was higher in Europe (96.9%) and lower in Africa (61.9%). Figure 2 shows the geographical distribution of HPV prevalence worldwide.
The prevalence of HPV by sex is shown in Table 2 and the virus was significantly more prevalent among women than among men p <0.0000001, OR: 3.27, 95% CI 2.26-4.74.
The presence of HPV DNA was associated with squamous cell carcinomas (p 0.0001, OR 9.05, 95% CI 5.37-15.3). Anal adenocarcinomas were investigated in five studies (31,40,45,56,58) (table 3). HPV 16 was the most prevalent genotype in all studies, followed by HPV 18 and 33; the prevalence of HPV 16 ranged from 70% to 93.7%. Multiple infections were detected in 11 of the 16 included studies. Table 4 shows the genotype distribution of HPV in anal cancers.
Detection and genotyping of HPV in colorectal cancer
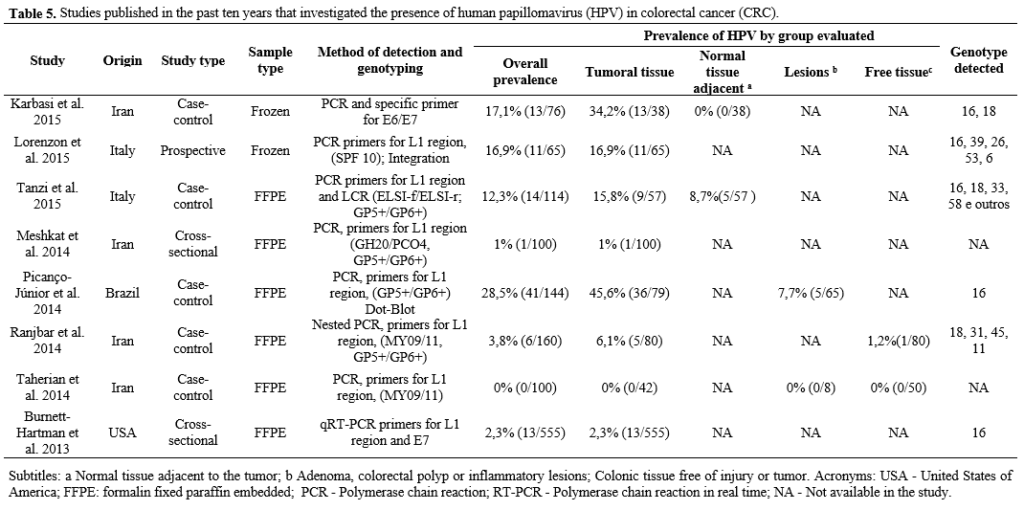
In total, 3992 colorectal samples were tested for HPV DNA (among tumors, lesions and neoplasia-free tissues) (11,17,26,28,29,34–39,42–44,47,49,51,53–55,59–64). Of these samples, 339 (8.5%. 95% CI 7.67-9.39) were positive for the presence of the virus, 10.9% in tumors, 5.5% in lesions, polyps and adenomas; 1.2% in tissue adjacent to the tumor free of neoplasia and 1% in healthy tissues. Of the 26 publications included, 11 investigated the presence of HPV only in colorectal tumors, without the presence of a control group (11,35,39,42,43,47,53,55,59–61). The study with the highest number of cases included a sample of 555 cases (2.3% HPV +) (39).
Most of the studies were case-control and used samples preserved in paraffin. The most used method for HPV detection was PCR and the most used primers were GP5 / 6 and MY09 / 11 (25,35–38,43,49,51,54,55,59,65).
HPV was more prevalent in Latin America (20.9%), followed by Asia (9.4%); North America (6.9%) and Europe (5.1%). The most detected genotype was HPV16 followed by HPV 18. Only one study did not find HPV 16 in colorectal carcinomas (37). The Table 5 presents the characteristics of the studies included.
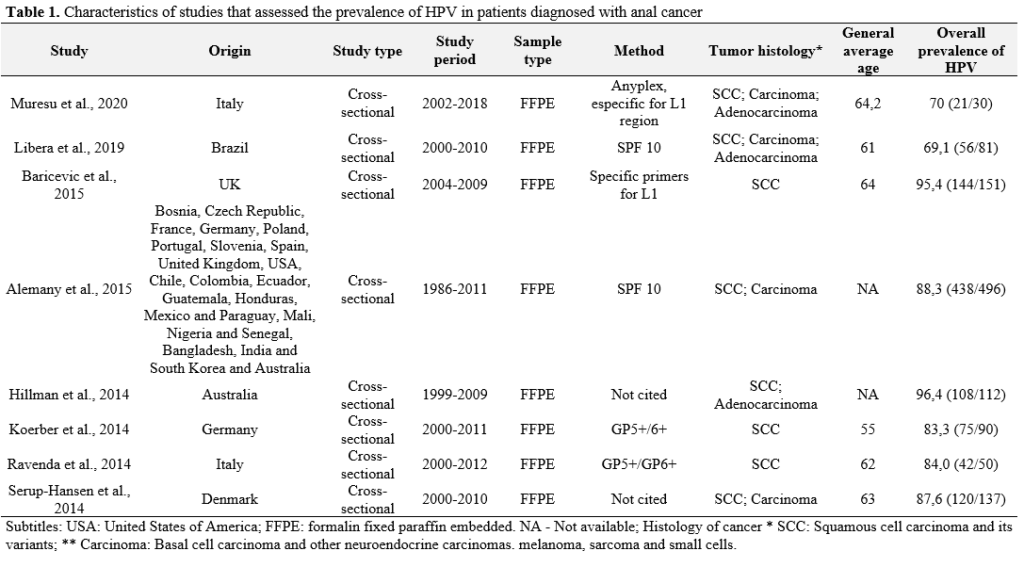


Figure 2. Geographical distribution of the number of cases tested for HPV and the prevalence of the virus by continent worldwide.
Table 2. Prevalence of HPV by sex in cases where the viral genome was detected.
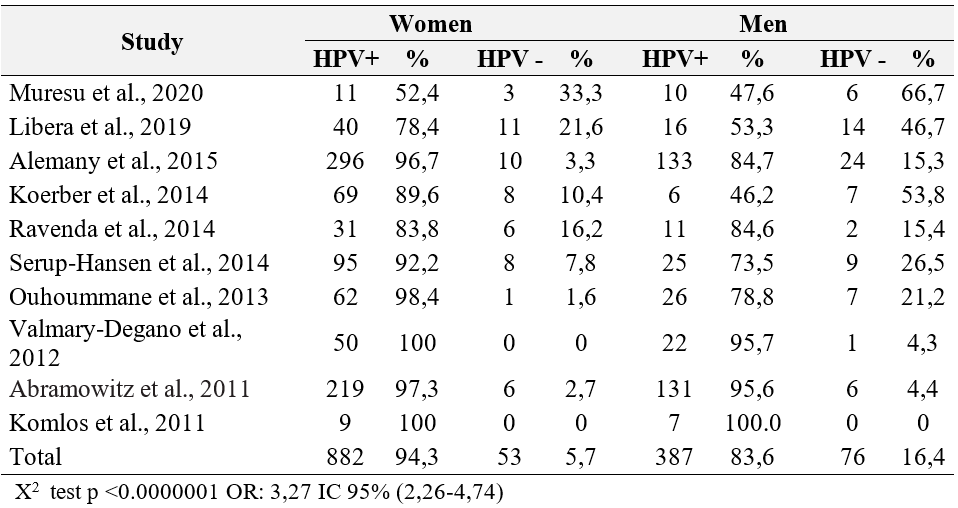
Table 3. Prevalence of HPV by histological type of anal cancer.


DISCUSSION
In the present review, HPV was detected in 88.9% of anal cancer samples, and in 10.9% of colorectal tumors. The high prevalence of HPV in anal tumors is well described in the literature, especially in squamous cell carcinomas (15,46). As demonstrated in the results, anal SCC rarely occurs in the absence of the virus, demonstrating an association between HPV and the development of anal cancer (p <0.0000001 OR: 9.08 95% CI 5.57-14.82).
In fact, anal carcinogenesis is very similar to that of the cervix, mainly because both anatomical sites have a zone with transitional epithelium. In addition, several factors contribute to HPV-induced anal cancer, such as early onset of sexual activity, sexual practices involving anal intercourse, previous exposure to high-risk HPV genotypes, a history of anogenital injury and other HPV associated cancers, especially in risky groups, such as men who have sex with men, transsexual women and individuals with the human immunodeficiency virus (HIV) (15).
In contrast, the role of HPV in colorectal cancer is not yet clear. Several authors have detected the virus in a portion of colon and rectal tumors and a few have described the presence of viral infection in both tumors,precursor lesions and normal colonic tissues (4,11–14,66). In order to establish the causal relationship between HPV and colorectal cancer, HPV DNA should be present in tumors and precursor lesions of CRC, and absent in healthy tissues. In this review, benign and precursor lesions for colorectal cancer such as polyps and adenomas had a prevalence of 5.5% of HPV DNA, while the normal adjacent tissue and healthy colonic tissue, a prevalence of less than 1.5 % was demonstrated, suggesting that there isn’t an association between HPV and colorectal cancer.
Some studies corroborate the idea that HPV is associated with CRC, as shown by the meta-analysis by Damin et al., 2013, carried out with 16 case-control studies that showed a general prevalence for the virus of 31.9% (95 CI %: 19.3-47.9) (12). However, the most complete meta-analysis appears to be that of Baandrup et al., 2014, which involved 37 publications and a total of 2630 colorectal adenocarcinomas, with a combined HPV prevalence of 11.2% (95% CI: 4.9-19.6). This same study showed a prevalence of HPV of 13.4% in normal tissue adjacent to the tumor; 1.6% in distant healthy tissues and 5.1% in adenomas (14).
A meta-analysis published in Brazil in 2016, in Portuguese, involved 1549 samples of colorectal cancers and described 18 included articles. The results demonstrate an HPV prevalence of 51.8% and a significant global effect (p <0.001), which led the authors to suggest an evident association between HPV and CRC (13). However, the criteria for evaluating the quality of the studies were not described in the study, nor even the guidelines employed for carrying out the systematic review and meta-analysis.
In Zhang meta-analysis (2018) only publications involving the Chinese population were included and the prevalence of the virus in tumor tissues and adenomas was higher than that observed in controls OR 10.78% (95% CI 4.22-27.53), high lighting the association of HPV with CCR in the Chinese population.
Both anal and colorectal tumors had a higher prevalence of HPV genotypes 16 and 18. These genotypes are considered high risk for carcinogenesis and are associated with different types of anogenital cancers (67). Currently, a prophylactic vaccine for the most common HPV genotypes (HPV 6, 11, 16 and 18) is available for most countries. The vaccine has the potential to prevent more than two-thirds of anal cancers in women and men, playing an important role in the primary prevention of these tumors in both sexes (7,21). For this reason, studies evaluating the prevalence of HPV genotypes in different regions are of great importance, making it possible to evaluate the effectiveness of the vaccine over the years.
Multiple infections were found in 11 of the 16 anal cancer studies included in this review (7,25,62,26,32,39–41,47,48,54). A study carried out in Australia tested 112 samples of anal cancer, of which 96.4% were positive for HPV, among which 23.2% tested for multiple infections (39). Risky sexual practices, such as multiple sexual partners, increased exposure to different HPV genotypes, in addition to the use of genotyping methodologies with a broad spectrum of HPV genotypes, can detect a greater number of genotypes (7). Through this review, it was not possible to evaluate the prognostic role of multiple infections in patients diagnosed with anal carcinomas, suggesting the need for research involving viral genotyping and investigating the associations between the clinical and pathological characteristics of each individual with the presence of simple or multiple infections.
Studies involving adenocarcinomas and other types of anal cancer did not show a significant association for HPV infection. In contrast to SCC, adenocarcinoma of the anus is not intrinsically related to HPV infection (68). These data suggest that more than half of anal adenocarcinomas are probably not related to HPV, which is also seen in colorectal cancer. However, although the virus does not show tropism for the glandular epithelium, studies are needed to investigate the etiological role of HPV in adenocarcinomas, such as studies of transcriptionally active infection or viral integration.
All detection methods of the studies included in this review are based on the polymerase chain reaction (PCR) technique and mainly use samples fixed in formaldehyde and preserved in paraffin. The most used primers for detection and genotyping were GP5 / 6 and MY09 / 11. By using several sets of specific primers in the same reaction (multiplex PCR) and amplifying fragments of different sizes for HPV genotyping, PCR is one of the most used methodologies for detecting HPV in paraffin material. The main primers used are PGMY09 / 11 (20 bp primers leading to a 450 bp amplicon); GP5 + / GP6 + primers (produce 150 bp amplicon and are more sensitive); and the SPF10 primers that lead to the amplification of a 65 bp viral DNA fragment (very sensitive detection) (69). As paraffinized samples tend to be more susceptible to DNA degradation compared to frozen or fresh samples (70), high performance detection tests are increasingly used, such as the commercial INNO-LiPA HPV Genotyping Extra II test (Fujirebio Europe ®), which is highly sensitive and allows the simultaneous detection of several genotypes in a single sample (71–73).
The prevalence of HPV in anal cancers was relatively higher in women (0.0000001 OR: 3.27 95% CI 2.26-4.74), which can potentially be explained by the persistence of the virus in the anal canal and by pre-existing in the cervix, vagina or vulva (52,54,67,74). A study evaluated 517 cases of anal cancer diagnosed over 13 years in women and showed that a history of grade 3 cervical intraepithelial neoplasia (NIC3), smoking, use of oral contraceptives, nulliparity and tubal ligation, are risk factors for anal cancer in women (75). Another possibility is that the anal mucosa may be sensitive to estrogenic action (76).
The difference in the prevalence of HPV infection has also been described in relation to geographical variation. The European continent had the highest number of cases of anal cancer and the highest prevalence of HPV (96.9%). Unlike another study that reported a higher prevalence of the virus in North America (7). The lowest prevalence of HPV and the lowest number of cases of anal cancer were observed in Africa (61.9%). However, we emphasize that this review did not include studies that assessed the prevalence of HPV in immunocompromised or HIV-infected individuals. Unfortunately, during data collection, most studies carried out in Africa showed specific populations that did not meet our inclusion criteria.
Differences in the prevalence of HPV in the CRC in relation to geographical location were not observed in this review. Among the studies analyzed, HPV investigated in CRCs was more prevalent in Latin America (20.9%). In two previous meta-analyzes (12,14), the prevalence of HPV infection was higher in South America, ranging from 32% to 45%, and the lowest prevalence (3% or less) was observed in North America, Europe and Australia (14). More recent publications (35,65) demonstrate higher prevalence of HPV infection in CRC in the European continent (12.3% to 16.9%) and in North America, respectively (23.8% to 42.2%) (28,64,77). Probably the differences observed in the prevalence of HPV infection in CRCs may be the result of methodological differences, sample preparation and tumor location (78).
HPV infection in anal and colorectal cancer can have variable prevalence and be influenced by the HPV detection method used. Some limitations can be observed for this review, for instance the heterogeneity of the studies, methodological differences such as sensitivity and specificity of the PCR methods used for HPV detection and genotyping, characteristics of the studied populations, variation in the histological diagnosis, anatomical location of the tumors and inadequate material. Even so, these data strongly support the association of HPV with anal cancer and the possible benefit of the anti-HPV vaccine in the population, in both sexes.
CONCLUSION
Anal cancers had a higher prevalence of HPV infection (89%) than colorectal cancers (8.5%), with genotypes 16 and 18 being the most commonly found. HPV was significantly associated with anal squamous cell carcinoma (p <0.0001) and was more prevalent in women with anal cancers (p <0.0001).
Given the similar histopathological characteristics between anal carcinoma and other anogenital carcinomas, in which HPV infection is the causative agent, HPV is likely to play the same etiological role in anal tumors, but not in colorectal cancers.
REFERENCES
1. IARC. Cancer Today: Colorectal cancer [Internet]. International Agency for Research on Cancer. World Health Organization. 2021 [cited 2020 Jan 1]. p. 1. Available from: https://gco.iarc.fr/today/home
2. Loukola A, Salovaara R, Kristo P, Moisio A, Ka H, Mecklin J. Microsatellite Instability in Adenomas as a Marker for Hereditary Nonpolyposis Colorectal Cancer. Am J Pathol. 1999;155(6):1849–53.
3. Morán A, Ortega P, Juan C De, Fernández-marcelo T, Frías C, Sánchez- A, et al. Differential colorectal carcinogenesis : Molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2(3):151–8.
4. Chen H, Chen XZ, Waterboer T, Castro FA, Brenner H. Viral infections and colorectal cancer: A systematic review of epidemiological studies. Int J Cancer. 2015;137(1):12–24.
5. Militello V, Trevisan M, Squarzon L, Biasolo MA, Rugge M, Militello C, et al. Investigation on the presence of polyomavirus, herpesvirus, and papillomavirus sequences in colorectal neoplasms and their association with cancer. Int J Cancer. 2009;124(10):2501–3.
6. Hartwig S, Syrjänen S, Dominiak-Felden G, Brotons M, Castellsagué X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: A review. BMC Cancer. 2012;12.
7. Alemany L, Saunier M, Alvarado I, Quirós B, Salmeron J, Shin H, et al. HPV DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer [Internet]. 2015;136(1):98–107. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4270372/pdf/nihms644742.pdf
8. Plummer M, Martel C de, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Heal. 2016;4(1):e609–16.
9. Crow JM. HPV: The global burden. Nature. 2012 Aug;488(7413):S2-3.
10. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70.
11. Lorenzon L, Mazzetta F, Pilozzi E, Uggeri G, Torrisi MR, Ferri M, et al. Human papillomavirus does not have a causal role in colorectal carcinogenesis. World J Gastroenterol. 2015;21(1):342–50.
12. Damin DC, Ziegelmann PK, Damin AP. Human papillomavirus infection and colorectal cancer risk: A Meta-analysis. Color Dis. 2013;15(8):420–8.
13. Pelizzer T, Dias CP, Poeta J, Torriani T, Roncada C. Colorectal cancer prevalence linked to human papillomavirus: A systematic review with meta-analysis. Rev Bras Epidemiol. 2016;19(4):791–802.
14. Baandrup L, Thomsen LT, Olesen TB, Andersen KK, Norrild B, Kjaer SK. The prevalence of human papillomavirus in colorectal adenomas and adenocarcinomas: A systematic review and meta-analysis. Eur J Cancer [Internet]. 2014;50(8):1446–61. Available from: http://dx.doi.org/10.1016/j.ejca.2014.01.019
15. Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–80.
16. Tota JE, Chevarie-Davis M, Richardson LA, DeVries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: Implications for prevention strategies. Prev Med (Baltim) [Internet]. 2011;53(SUPPL. 1):S12–21. Available from: http://dx.doi.org/10.1016/j.ypmed.2011.08.017
17. Vuitton L, Jaillet C, Jacquin E, Monnien F, Heberle M, Mihai MI, et al. Human papillomaviruses in colorectal cancers: A case-control study in western patients. Dig Liver Dis [Internet]. 2017;49(4):446–50. Available from: http://dx.doi.org/10.1016/j.dld.2016.11.003
18. Burnett-Hartman AN, Feng Q, Popov V, Kalidindi A, Newcomb PA. Human papillomavirus DNA is rarely detected in colorectal carcinomas and not associated with microsatellite instability: the Seattle colon cancer family registry. Cancer Epidemiol biomarkers Prev. 2013 Feb;22(2):317–9.
19. Chen; H, Chen X-Z, Waterboer T, Castro FA, Brenner H. Viral infections and colorectal cancer: a systematic review of epidemiological studies. Int J Cancer. 2015;137(1):12–24.
20. Zhang XH, Wang W, Wang YQ, Jia DF, Zhu L. Human papillomavirus infection and colorectal cancer in the Chinese population: a meta-analysis. Color Dis. 2018;20(11):961–9.
21. Dadar M, Chakraborty S, Dhama K, Prasad M, Khandia R, Hassan S, et al. Advances in Designing and Developing Vaccines, Drugs and Therapeutic Approaches to Counter Human Papilloma Virus. Front Immunol [Internet]. 2018;9(2478):1–32. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2018.02478/full
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg [Internet]. 2010;8(5):336–41. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1743919110000403
23. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(1):1495–9.
24. Dalla Libera LS, de Siqueira T, Santos IL, Porto Ramos JE, Milhomen AX, de Cassia Gonçalves de Alencar R, et al. Detection of Human papillomavirus and the role of p16INK4a in colorectal carcinomas. PLoS One. 2020;15(6 June):1–22.
25. Muresu N, Sotgiu G, Saderi L, Sechi I, Cossu A, Marras V, et al. Distribution of hpv genotypes in patients with a diagnosis of anal cancer in an Italian region. Int J Environ Res Public Health. 2020;17(12):1–9.
26. Libera LSD, Carvalho KPA De, Ramos JEP, Cabral LAO, Alencar RDCG De, Villa LL, et al. Human Papillomavirus and Anal Cancer: Prevalence, Genotype Distribution, and Prognosis Aspects from Midwestern Region of Brazil. J Oncol. 2019;1(1):1–11.
27. Biesaga B, Janecka-Widła A, Kołodziej-Rzepa M, Słonina D, Darasz Z, Gasińska A. The prevalence of HPV infection in rectal cancer – Report from South – Central Poland (Cracow region). Pathol Res Pract. 2019;215(9):1–5.
28. Shamir ER, Devine WP, Pekmezci M, Umetsu SE, Krings G, Federman S, et al. Identification of high-risk human papillomavirus and Rb/E2F pathway genomic alterations in mutually exclusive subsets of colorectal neuroendocrine carcinoma. Mod Pathol [Internet]. 2019;32(2):290–305. Available from: http://dx.doi.org/10.1038/s41379-018-0131-6
29. Afshar RM, Deldar Z, Mollaei HR, Arabzadeh SA, Iranpour M. Evaluation of HPV DNA positivity in colorectal cancer patients in Kerman, Southeast Iran. Asian Pacific J Cancer Prev. 2018;19(1):193–8.
30. Gazzaz F, Mosli MH, Jawa H, Sibiany A. Detection of human papillomavirus infection by molecular tests and its relation to colonic polyps and colorectal cancer. Saudi Med J. 2016;37(3):256–61.
31. Soto Y, Limia CM, González L, Grá B, Hano OM, Martínez PA, et al. Molecular evidence of high-risk human papillomavirus infection in colorectal tumours from cuban patients. Mem Inst Oswaldo Cruz. 2016;111(12):731–6.
32. Baricevic I, He X, Chakrabarty B, Oliver AW, Bailey C, Summers J, et al. High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: Different implications for vaccine prevention and prognosis. Eur J Cancer [Internet]. 2015;51(6):776–85. Available from: http://dx.doi.org/10.1016/j.ejca.2015.01.058
33. Bernabe-Dones RD, Gonzalez-Pons M, Villar-Prados A, Lacourt-Ventura M, Rodríguez-Arroyo H, Fonseca-Williams S, et al. High Prevalence of Human Papillomavirus in Colorectal Cancer in Hispanics: A Case-Control Study. Gastroenterol Res Pract. 2016;1(1):1–8.
34. Karbasi A, Borhani N, Daliri K, Kazemi B, Manoochehri M. Downregulation of external death receptor genes FAS and DR5 in colorectal cancer samples positive for human papillomavirus infection. Pathol Res Pract [Internet]. 2015;211(6):444–8. Available from: http://dx.doi.org/10.1016/j.prp.2015.02.001
35. Lorenzon L, Mazzetta F, Pilozzi E, Uggeri G, Torrisi MR, Ferri M, et al. Human papillomavirus does not have a causal role in colorectal carcinogenesis. World J Gastroenterol. 2015;21(1):342–50.
36. Mahmoudvand S, Safaei A, Erfani N, Sarvari J. Presence of human papillomavirus DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Asian Pacific J Cancer Prev. 2015;16(17):7883–7.
37. Tanzi E, Bianchi S, Frati ER, Amicizia D, Martinelli M, Bragazzi NL, et al. Human papillomavirus detection in paraffin-embedded colorectal cancer tissues. J Gen Virol. 2015;96(1):206–9.
38. Koerber SA, Schoneweg C, Slynko A, Krug D, Haefner MF, Herfarth K, et al. Influence of human papillomavirus and p16INK4a on treatment outcome of patients with anal cancer. Radiother Oncol [Internet]. 2014;113(3):331–6. Available from: http://dx.doi.org/10.1016/j.radonc.2014.11.013
39. Hillman RJ, Garland SM, Gunathilake MPW, Stevens M, Kumaradevan N, Lemech C, et al. Human papillomavirus (HPV) genotypes in an Australian sample of anal cancers. Int J Cancer. 2014;135(4):996–1001.
40. Ravenda PS, Magni E, Botteri E, Manzotti M, Barberis M, Vacirca D, et al. Prognostic value of human papillomavirus in anal squamous cell carcinoma. Cancer Chemother Pharmacol. 2014;74(5):1033–8.
41. Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, Hgødall E, Geertsen PF, Havsteen H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32(17):1812–7.
42. Meshkat M, Tayyebi Meibodi N, Sepahi S, Fadaee N, Salehpour M, Meshkat Z. The frequency of human papillomaviruses in colorectal cancer samples in Mashhad northeastern Iran. Turkish J Med Sci. 2014;44(3):501–3.
43. Picanço-Junior OM agalhãe., Oliveira AL ui. T, Freire LT herez. M, Brito RB ai., Villa LL in., Matos D. Association between human Papillomavirus and colorectal adenocarcinoma and its influence on tumor staging and degree of cell differentiation. Arq Bras Cir Dig. 2014;27(3):172–6.
44. Ranjbar R, Saberfar E, Shamsaie A, Ghasemian E. The aetiological role of human papillomavirus in colorectal carcinoma: An Iranian population- based case control study. Asian Pacific J Cancer Prev. 2014;15(4):1521–5.
45. Taherian H, Tafvizi F, Fard ZT, Abdirad A. Lack of association between human papillomavirus infection and colorectal cancer. Prz Gastroenterol. 2014;9(5):280–4.
46. Burnett-Hartman AN, Feng Q, Popov V, Kalidindi A, Newcomb PA. Human papillomavirus DNA is rarely detected in colorectal carcinomas and not associated with microsatellite instability: The Seattle Colon Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2013;22(2):317–9.
47. Ouhoummane N, Steben M, Coutlée F, Vuong T, Forest P, Rodier C, et al. Squamous anal cancer: Patient characteristics and HPV type distribution. Cancer Epidemiol. 2013;37(6):807–12.
48. Valmary-Degano S, Jacquin E, Prétet JL, Monnien F, Girardo B, Arbez-Gindre F, et al. Signature patterns of human papillomavirus type 16 in invasive anal carcinoma. Hum Pathol [Internet]. 2013;44(6):992–1002. Available from: http://dx.doi.org/10.1016/j.humpath.2012.08.019
49. Chen TH, Huang CC, Yeh KT, Chang SH, Chang SW, Sung WW, et al. Human papilloma virus 16 E6 oncoprotein associated with p53 inactivation in colorectal cancer. World J Gastroenterol. 2012;18(30):4051–8.
50. Ghabreau L, Segal E, Yasmeen A, Kassab A, Akil N, Al Moustafa A-E. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: A tissue microarray study. Clin Cancer Investig J. 2012;1(1):26.
51. Snietura M, Waniczek D, Nowakowska-Zajdel E, Piglowski W, Kopec A, Muc-Wierzgon M. Does human papilloma virus participate in colorectal carcinogenesis? J Biol Regul Homeost Agents. 2012;26(4):757–62.
52. Abramowitz L, Jacquard AC, Jaroud F, Haesebaert J, Siproudhis L, Pradat P, et al. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer. 2011;129(2):433–9.
53. Karpinski P, Myszka A, Ramsey D, Kielan W, Sasiadek MM. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumor Biol. 2011;32(4):653–9.
54. Komlos FK, Kocjan BJ, Kosorok P, Rus T, Toplak J, Bunic M, et al. Distribution of HPV genotypes in Slovenian patients with anal carcinoma: Preliminary results. Acta Dermatovenerologica Alpina, Pannonica Adriat. 2011;20(3):141–3.
55. Liu F, Mou X, Zhao N, Lin J, Teng L, Xiang C. Prevalence of human papillomavirus in Chinese patients with colorectal cancer. Color Dis. 2011;13(8):865–71.
56. Soares PC, Ferreira S, Villa LL, Matos D. Identificação do papilomavírus humano em doentes com carcinoma de células escamosas do canal anal e sua relação com ograu de diferenciação celular e estadiamento Paulo Cardoso Soares e Cols. Rev Bras Coloproctol [Internet]. 2011;31(1):8–16. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-98802011000100002
57. Yavuzer D, Karadayi N, Salepci T, Baloglu H, Dabak R, Bayramicli OU. Investigation of human papillomavirus DNA in colorectal carcinomas and adenomas. Med Oncol. 2011;28(1):127–32.
58. Yhim HY, Lee NR, Song EK, Kwak JY, Lee ST, Kim JH, et al. The prognostic significance of tumor human papillomavirus status for patients with anal squamous cell carcinoma treated with combined chemoradiotherapy. Int J Cancer. 2011;129(1):1752–60.
59. Deschoolmeester V, Van Marck V, Baay M, Weyn C, Vermeulen P, Van Marck E, et al. Detection of HPV and the role of p16INK4A overexpression as a surrogate marker for the presence of functional HPV oncoprotein E7 in colorectal cancer. BMC Cancer. 2010;10(117):1–10.
60. Gornick MC, Castellsague X, Sanchez G, Giordano TJ, Michelle Vinco, Greenson JK, et al. Human papillomavirus is not associated with colorectal cancer in a large international study. Cancer Causes Control. 2010;21(5):737–43.
61. Pérez LO, Barbisan G, Ottino A, Pianzola H, Golijow CD. Human papillomavirus DNA and oncogene alterations in colorectal tumors. Pathol Oncol Res. 2010;16(3):461–8.
62. Ramamoorthy S, Liu YT, Luo L, Miyai K, Lu Q, Carethers JM. Detection of multiple human papillomavirus genotypes in anal carcinoma. Infect Agent Cancer [Internet]. 2010;5(17):1–5. Available from: http://www.infectagentscancer.com/content/5/1/17
63. Wong AK, Chan RC, Aggarwal N, Singh MK, Nichols WS, Bose S. Human papillomavirus genotypes in anal intraepithelial neoplasia and anal carcinoma as detected in tissue biopsies. Mod Pathol [Internet]. 2010;23(1):144–50. Available from: http://dx.doi.org/10.1038/modpathol.2009.143
64. R.D. B-D, M. G-P, A. V-P, M. L-V, H. R-A, S. F-W, et al. High Prevalence of Human Papillomavirus in Colorectal Cancer in Hispanics: A Case-Control Study. Gastroenterol Res Pract [Internet]. 2016;2016:no pagination. Available from: http://www.hindawi.com/journals/grp/%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed13&NEWS=N&AN=20160148006
65. Tanzi E, Bianchi S, Frati ER, Amicizia D, Martinelli M, Bragazzi NL, et al. Human papillomavirus detection in paraffin-embedded colorectal cancer tissues. J Gen Virol. 2015;96(1):206–9.
66. EDGE; SB, BYRD; DR, COMPTON; CC, FRITZ; AG, GREENE; FL, ANDY TROTTI I. AJCC Cancer Staging Manual. 7th ed. American Joint Committee on Cancer. Chicago, EUA; 2010. 672 p.
67. De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int J Cancer. 2009;124(7):1626–36.
68. Jin F, Stein AN, Conway EL, Regan DG, Law M, Brotherton JML, et al. Trends in anal cancer in Australia, 1982-2005. Vaccine [Internet]. 2011;29(1):2322–7. Available from: http://dx.doi.org/10.1016/j.vaccine.2011.01.015
69. Eide ML, Debaque H. HPV detection methods and genotyping techniques in screening for cervical cancer. Ann Pathol. 2012 Dec;32(6):e15-23, 401–9.
70. Steinau M, Patel SS, Unger ER. Efficient DNA extraction for HPV genotyping in formalin-fixed, paraffin-embedded tissues. J Mol Diagnostics [Internet]. 2011;13(4):377–81. Available from: http://dx.doi.org/10.1016/j.jmoldx.2011.03.007
71. Alberizzi P, Spinillo A, Gardella B, Cesari S, Silini EM. Evaluation of the HPV typing INNO-LiPA EXTRA assay on formalin-fixed paraffin-embedded cervical biopsy samples. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2014 Dec;61(4):535–9.
72. Kocjan BJ, Seme K, Poljak M. Comparison of the Abbott RealTime High Risk HPV test and INNO-LiPA HPV Genotyping Extra test for the detection of human papillomaviruses in formalin-fixed, paraffin-embedded cervical cancer specimens. J Virol Methods. 2011 Jul;175(1):117–9.
73. Sohrabi A, Rahnamaye-Farzami M, Mirab-Samiee S, Mahdavi S, Babaei M. Comparison of In-House Multiplex Real Time PCR, Diagcor GenoFlow HPV Array Test and INNO-LiPA HPV Genotyping Extra Assays with LCD- Array Kit for Human Papillomavirus Genotyping in Cervical Liquid Based Cytology Specimens and Genital Lesions in Tehran, I. Clin Lab. 2016;62(4):615–9.
74. Tachezy R, Jirasek T, Salakova M, Ludvikova V, Kubecova M, Horak L, et al. Human papillomavirus infection and tumours of the anal canal: correlation of histology, PCR detection in paraffin sections and serology. APMIS. 2007;115(1):195–203.
75. Coffey K, Beral V, Green J, Reeves G, Barnes I. Lifestyle and reproductive risk factors associated with anal cancer in women aged over 50 years. Br J Cancer [Internet]. 2015;112(1):1568–74. Available from: http://dx.doi.org/10.1038/bjc.2015.89
76. Rizk DEE, Hassan HA, Ramadan GA, Shafiullah M, Fahim MA. Estrogen and ghrelin increase number of submucosal urethral and anal canal blood vessels in ovariectomized rats. Urology. 2005;66(6):1343–8.
77. Soto D, Song C, McLaughlin-Drubin ME. Epigenetic alterations in human papillomavirus- associated cancers. Viruses. 2017;9(9).
78. Lorenzon L, Ferri M, Pilozzi E, Torrisi MR, Ziparo V, French D. Human papillomavirus and colorectal cancer: Evidences and pitfalls of published literature. Int J Colorectal Dis. 2011;26(2):135–42.
1Universidade Federal de Goiás, Programa de Pós-Graduação em Ciências da Saúde, Faculdade de Medicina, Goiânia, Goiás, CEP 74605-020, Brasil. larisse_dalla@hotmail.com. MD, Universidade Federal de Goiás, Anápolis, Goiás, CEP 75130-670, Brasil. Telephone number: +55-62-993298343. Collaboration in the manuscript: All stages, from search and selection of articles, to the discussion of results. She also performed the statistical analysis.
2Pontificia Universidade Católica de Goiás, Goiânia, Programa de Pós-Graduação em Ciências Ambientais e Saúde, Goiás, CEP 74175-120, Brasil. jessicaenocencio14@gmail.com. Collaboration in the manuscript: All stages, from search and selection of articles, to the discussion of results.
3Pontificia Universidade Católica de Goiás, Goiânia, Programa de Pós-Graduação em Ciências Ambientais e Saúde, Goiás, CEP 74175-120, Brasil. igorlsantoss@outlook.com. Collaboration in the manuscript: Discussion of results.
4Universidade Federal de Goiás, Goiânia, Programa de Pós-Graduação em Ciências da Saúde, Faculdade de Medicina, Goiás, CEP 74605-020, Brasil. verasaddi@gmail.com. Collaboration in the manuscript: Orientation, supervision and correction of the manuscript.
