REGISTRO DOI:10.69849/revistaft/th102410031639
Jose Alves Rodrigues 1,
Amanda Santoro Fonseca Bacchin 2
Marcela Cassol 3
Rodrigo Rodrigues Virgolino 4
Daniele Salgado de Sousa 5
Ysadora Maria Rodrigues Pinto 6
Vitor Hugo Auzier Lima 7
ABSTRACT
Introduction: Heart failure (HF) is the leading cause of death in the world and can be defined as a complex syndrome in which the heart is unable to pump enough blood to meet the body’s metabolic needs; or can do so only at the expense of elevations in filling pressure. Objective: To assess whether patients at the Hospital do Servidor Público Municipal de São Paulo with heart failure, regardless of ejection fraction, are using ISGLT2. Methods: This is an observational, descriptive, and cross-sectional study conducted between November 2023 and April 2024. The sample of this study included 65 patients with heart failure documented by echocardiogram and electronic medical record, of both sexes, over 18 years of age . Patients were subjected to a telephone interview, in which they were asked about their regular use of the medication dapagliflozin; which specialty they were clinically followed up with; and if they were not using it, the reason for not taking it was investigated. Methods: This is an observational, descriptive, and cross-sectional study carried out between November 2023 and April 2024. The sample of this study included 65 patients with heart failure documented through echocardiogram and electronic medical records, of both sexes, over 18 years of act. Patients were subjected to a telephone interview, in which they were asked about their regular use of the medication dapagliflozin; which specialty they were clinically followed up with; and if they were not using it, the reason for not taking it was investigated.Results: The sample consisted of men (46%) and women (54%) between 40 and 91 years old, with a mean age of 74.4 years and preserved ejection fraction (mean 56.5%). Of these, 58.5% (38 patients) were diabetic and only 23.1% used dapagliflozin regularly. Among the patients who used the medication, cardiology and endocrinology (33% each) were the specialties that prescribed the drug the most, followed by geriatrics. Among the 76.9% of patients who were not using the medication, the main reason given was the lack of a prescription for the drug (74%); only 2% stopped using it due to adverse effects and another 2% did not even use it, despite being prescribed, for fear of side effects. Discussion: Although dapagliflozin is a medication recommended by the main societies for the treatment of not only heart failure, but also diabetes and nephropathy, the low number of individuals using dapagliflozin (23.1%) is noteworthy. Were their respective doctors unable to clarify and address their doubts during consultations? Did the patients not feel comfortable asking questions? These questions call into question the quality of our consultations and the fragile doctor-patient relationship. Conclusion: Sodium-glucose cotransporter 2 inhibitors have demonstrated several clinical benefits, as reported in the literature. Raising awareness of this issue among specialist and general practitioner health professionals is of utmost importance, given the high mortality and morbidity rates among this group of patients. The medication is already available at popular pharmacies in Brazil at a discount and free of charge for certain groups at high-cost government pharmacies. It may be necessary to ensure that general practitioners have access to updated information and to review the way in which we address our patients’ concerns and concerns.
Key Words: Heart Failure. Isglt2. Dapaglifozin.
1. INTRODUCTION
1.1. Epidemiology
Heart failure (HF) is the leading cause of death in the world and can be defined as a complex syndrome in which the heart is unable to pump enough blood to meet the body’s metabolic needs; or can do so only at the expense of elevated blood pressure. filling pressure. It is characterized by typical signs and symptoms resulting from reduced cardiac output and/or elevated filling pressures at rest or during exercise. ( 1)
The global prevalence is 64 million people, with 6 million in the Americas alone. It is estimated that by 2030 there will be 8 million, which represents a 46% increase in the number of people living with this disease. ( 2)
Heart failure can currently be considered a global epidemic with high morbidity and mortality. New revolutionary drugs capable of reducing complications, mortality and hospitalizations have been discovered, providing an improvement in the quality of life of patients. This is the case of sodium glucose cotransporter type 2 inhibitors (SGLT2i), which have already been shown to be capable of reducing hospitalization for HF, regardless of the presence of diabetes and ejection fraction, and are the drug with the greatest therapeutic benefit found to date. ( 3)
In randomized clinical trials, this class of drugs has also demonstrated benefits in renal outcomes. Early studies showed favorable results in patients with HF with reduced ejection fraction; later it was also demonstrated that the positive effects extended to patients with preserved ejection fraction. ( 4) However, despite therapeutic advances in recent decades, mortality remains around 50% in 5 years. In Brazil alone, mortality from heart failure between 2018 and 2019 reached 12,000 deaths per year among elderly people over 80 years of age. ( 5)
It is a serious and very common syndrome, affecting approximately 64 million people worldwide. It is estimated that 4 million patients currently have HF in Brazil. Approximately 50% of all patients hospitalized for HF are readmitted within 90 days after hospital discharge. It is estimated that 50% of patients with HF will die within 5 years after diagnosis, and the prevalence increases with age. (6)
Recent data have shown that late mortality among patients with chronic HF is classified by ejection fraction (EF), reaching the highest rate in patients with heart failure with reduced ejection fraction (HFrEF) (8.8%), followed by heart failure with mildly reduced ejection fraction (HFmrEF) (7.6%) and heart failure with preserved ejection fraction (HFpEF) (6.3%). In Brazil, hospital mortality is high, one of the highest in the Western world, and low adherence to specific treatments for heart failure is one of the main causes of readmissions. (7)
Ischemic causes are the most common worldwide. In our country, poor control of comorbidities such as chronic kidney disease (CKD), systemic arterial hypertension (SAH) and type 2 diabetes mellitus (DM2) have led to the frequent occurrence of this syndrome. (7)
Cardiovascular (CV) and renal diseases are connected, causing and resulting from each other; and should be treated together. It is known that CKD worsens the prognosis of CV diseases. The risk of hospitalization and the risk of death from all causes increase with the progression of kidney disease. CKD is a highly prevalent disease, affecting more than 843 million people, and its main causes are cardiometabolic in origin. ( 8)
Pharmacological treatment aims to reduce morbidity and mortality and improve quality of life. The drugs that have these properties are angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), angiotensin receptor neprilysin inhibitors (ARNIs), beta-blockers, mineralocorticoid receptor antagonists (MRA), ISGLT2, hydralazine combined with nitrate and ivabradine.
1.2 ISGLT2
The SGLT2 receptor is found in the renal proximal tubule and is responsible for the reabsorption of nearly all glucose and most of the sodium filtered in the glomerulus. Inhibition of this receptor produces natriuresis and glycosuria, providing an insulin-independent method of reducing blood glucose without the risk of hypoglycemia. In addition to glycemic control, it has other pleiotropic properties, including effects on body weight, blood pressure, and lipids. Studies of empagliflozin, canagliflozin, and dapagliflozin have been shown to reduce hospitalizations for HF in patients with diabetes and high cardiovascular risk and also reduce the progression of kidney disease. DAPA-HF was the first randomized clinical trial to investigate the impact of an SGLT2 inhibitor ( Figure 1) in patients with HFrEF, regardless of the diagnosis of diabetes. ( 10)
Figure 1: Key effects of ISGLT2.
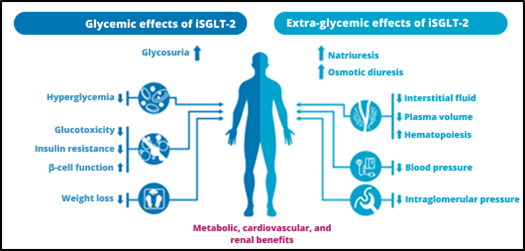
Source: Adapted from Heerpink et al. (2018).
1.3 Glycemic effects of ISGLT2
SGLT2 cotransporters are expressed almost exclusively in the kidneys. They are present in the first portion of the proximal convoluted tubule and are responsible for 90% of glucose reabsorbed in the kidneys. For each sodium molecule reabsorbed by SGLT2 receptors, one glucose molecule is reabsorbed. Glycosuria can usually be identified when serum glucose levels exceed 180 mg/dL. However, in individuals with diabetes, this cotransporter is upregulated, causing glycosuria to only occur at levels close to 250 mg/dL. The use of ISGLT2 reduces the renal glucose excretion threshold to 80-90 mg/dL, inducing the patient to present glycosuria at these levels. (Figure 2) . In normoglycemic patients, glycosuria induced by these medications is reduced, so the risk of hypoglycemia is low. In terms of hypoglycemic potency, it is inferior to metformin or sulfonylureas, but it reduces glycated hemoglobin by between 0.5 and 1%. ( 11)
Weight loss usually occurs in the first 6 months of treatment and then stabilizes, resulting in an average weight loss of 2-3 kg. Glycosuria can generate a caloric deficit of up to 280 kcal per day and cause the individual to have increased hunger in the long term, which is why weight loss is not sustained. ( 12)
Figure 2: Mechanism of action of Dapagliflozin.
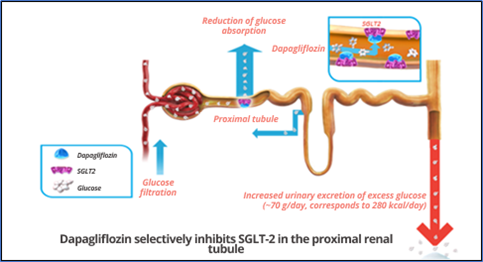
Source : Adapted from Wright (2001).
1.4 Extra-glycemic effects of ISGLT2
ISGLT2s cause an average 3 to 5 mmHg drop in systolic blood pressure (SBP) without increasing heart rate. One of the mechanisms by which this supposedly occurs is a reduction in sympathetic tone . ( 13)
Furthermore, these drugs increase the metabolism of ketones and free fatty acids by the heart, which appears to lead to improved energy efficiency. The consumption of ketone bodies also appears to exert anti-inflammatory activity. This metabolic effect associated with increased reabsorption of magnesium and potassium in the kidneys could be responsible for the effect of ISGLT2 in reducing sudden death. Hyperglycemia itself is linked to the activation of the renin-angiotensin-aldosterone system (RAAS), which in turn can cause left ventricular hypertrophy and myocardial fibrosis. Studies show that ISGLT2 can also reduce this activation of the RAAS. ( 13)
By reducing sodium and glucose absorption in the proximal convoluted tubule, it increases sodium availability in the distal convoluted tubule, where the macula densa is located. This causes vasoconstriction of the afferent arteriole, which reduces intraglomerular pressure and reduces glomerular hyperfiltration, reducing hemodynamic stress (tubuloglomerular feedback). As with ACE inhibitors, in the first few weeks there may be a slight worsening of renal function due to this rebalancing. An average reduction of 3 to 5 mL/min/1.73 m² is reported during this period. After this, renal function stabilizes and ISGLT2 reduces the progression of nephropathy. ( 14)
1.5 ISGLT2 Protection Mechanism
Although the exact mechanism of cardiorenal and HF protection by ISGLT2 has not been fully elucidated, potential mechanisms include improved glycemic control, weight loss, and reduced blood pressure; in addition to metabolic reprogramming causing the heart and kidneys to use lipids and ketones instead of carbohydrates. Also noteworthy is the influence of natriuresis and vascular function to optimize ventricular overload; renal hemodynamic regulation with correction of hyperfiltration, proteinuria, and hypoxia; which contribute to the reduction of inflammation and oxidative stress, thus producing antifibrotic effects; and improvement of mitochondrial function and autophagy (Figure 3). ( 14)
One of the most accepted mechanisms to explain the mode of action of ISGLT2 in heart failure is the improvement in left ventricular wall tone, secondary to the reduction of preload (effects of natriuresis and osmotic diuresis) and afterload (improvements in endothelial function and reduction in blood pressure). ( 14,15)
The potential renal protection mechanisms of ISGLT2 are: reduction of intraglomerular pressure, with stabilization of the estimated glomerular filtration rate (EGFR) and reduction of albuminuria; neurohormonal regulation, reducing blood pressure; inflammation and fibrosis. ( 15)
Figure 3: ISGLT2 protection mechanisms.
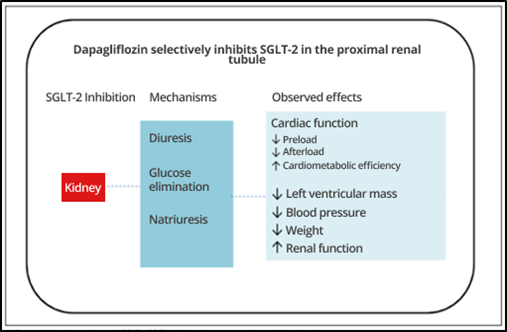
Source : Adapted from Bochi et al. (2021).
1.6 Safety and Adverse Effects of ISGLT2
The incidence of urinary tract and genital infections may increase during the use of ISGLT2 due to increased glycosuria, which predisposes to bacterial proliferation. However, given the benefits of these medications and the ease of treating these infections, their deprescription is not recommended unless these infections recur. ( 15,16)
ISGLT2 increases the risk of genital and/or urinary tract infections, but the absolute risk is low and, given the benefits of these drugs and the ease of treating genital and/or urinary tract infections, continued treatment is recommended unless these infections recur. The incidence of urinary tract and genital infections occurs due to increased glucose levels in these areas, which predisposes to bacterial proliferation. ( 16)
Studies have shown a greater than 2-fold increased risk of diabetic ketoacidosis (DKA) with SGLT-2 inhibitors, and this has been attributed to their effect in suppressing insulin secretion and increasing ketogenesis. However, the baseline risk of DKA was low in all trials, and simple measures to discontinue SGLT2 inhibitor use in patients taking insulin or secretagogues if food or medication intake is altered (e.g., surgery or diarrheal illness) may further mitigate this risk. Other adverse effects such as bone fracture, hypoglycemia, Fournier gangrene, volume depletion, and thromboembolic events were not relevant at the end of the studies. ( 16,17)
1.7 USE OF ISGLT2 IN PATIENTS WITH HF
ISGLT2s have shown robust results in both prevention and treatment of HF. Initial studies (EMPA-REG, CANVAS, DECLAIRE) with patients with T2DM initially aimed to demonstrate the cardiovascular safety of the drug, with benefits observed in HF in these patients. Based on these studies, new studies were designed to investigate the reduction in mortality in patients with HF regardless of the presence of diabetes. ( 17)
That said, this study attempted to assess whether patients with HF are receiving optimized therapy, including the prescription of this drug. And in cases where the occurrence of its prescription was low, we attempted to understand the reasons for this: Previous polypharmacy? Difficulty in accessing the medication? Adverse effects? Advanced age? Do only specialists have the habit of prescribing it? Furthermore, as the guiding principle of this research, this study aimed to assess whether patients at the Hospital do Servidor Público Municipal de São Paulo, with heart failure, regardless of ejection fraction, were using ISGLT2, as well as, secondarily, among the patients who were not receiving optimized therapy for CHF, we sought to identify the reasons why ISGLT-2 was not prescribed.
2. THEORETICAL FRAMEWORK
2.1 EMPA-REG Study
This study evaluated the role of empagliflozin in reducing cardiovascular risk in patients with T2DM. It was a randomized study that included patients with T2DM, > 18 years, body mass index (BMI) < 45, creatinine clearance (CrCl) > 30 mL/min/1.73m², with overt cardiovascular disease, and glycated hemoglobin (HbA1c) > 7%. These patients were divided into 3 groups: one received placebo, another received empagliflozin 10 mg once a day, and a third received empagliflozin 25 mg once a day. The primary outcome was death from cardiovascular causes, nonfatal acute myocardial infarction (AMI), and nonfatal stroke. The study results showed a decrease in the primary outcome (14% 0.86, CI 0.74 – 0.99) in the empagliflozin group, a decrease in cardiovascular mortality and overall mortality, and a decrease in hospitalizations for heart failure. There was no relevant difference in outcomes when compared with the two doses of empagliflozin. Thus, in diabetic patients at high risk of cardiovascular events, empagliflozin was associated with a lower combined risk of cardiovascular death or hospitalization for heart failure and a slower rate of progression of HF. ( 18)
2.2 CANVAS Study
The present study evaluated diabetic patients at high cardiovascular risk, randomized into 3 groups: canagliflozin 100 mg once a day, canagliflozin 300 mg once a day and placebo. The inclusion criteria were T2DM patients with HbA1c > 7% and < 10.5%, > 30 years with a history of symptomatic atherosclerotic cardiovascular disease or > 50 years with 2 or more of the following risk factors: T2DM for at least 10 years, systolic blood pressure (SBP) > 140 during treatment, smoker, microalbuminuria or macroalbuminuria. The primary outcome was a composite of death from cardiovascular causes, non-fatal MI and non-fatal stroke. The result was a reduction in the rate of the primary outcome (14% 0.86, CI 0.75 – 0.97) in the canagliflozin group. A reduction (22% 0.78, CI 0.67 – 0.91) was also observed in the composite of hospitalizations for heart failure and cardiovascular death (secondary objective). ( 18)
2.3 DECLARE TIMI 58 Study
Evaluated dapagliflozin in diabetic patients and the primary safety endpoint was a composite of cardiovascular death, MI or stroke (MACE) and the primary efficacy endpoints were MACE and a composite of cardiovascular death or hospitalization for heart failure. Patients were included with: age ≥ 40 years, T2DM with HbA1c between 6.5% and 12%, CrCl > 60 mL/min, previous cardiovascular event or multiple CV risk factors. These include women ≥ 60 years or men ≥ 55 years who had one of the following: SAH, dyslipidemia and smoking. Patients were randomized to receive placebo or dapagliflozin 10 mg once daily. The results showed non-inferiority of dapagliflozin compared to placebo with respect to the primary safety endpoint (MACE). Dapagliflozin did not result in a significantly lower incidence of MACE, but did result in a significantly lower rate of cardiovascular death (2% 0.98, CI 0.82–0.17) or hospitalization for heart failure (17% 0.83, CI 0.73–0.95) than placebo, with the additional finding of a possible lower rate of adverse renal outcomes. ( 19)
2.4 DAPA-HF Study
DAPA-HF randomized patients with symptomatic HFrEF, defined as ejection fraction (EF) ≤ 40% and New York Heart Association (NYHA) functional class ≥ II, to dapagliflozin 10 mg once daily or placebo, regardless of prior diabetes diagnosis; all participants received best available evidence-based treatment, with dapagliflozin being the only difference between groups. The majority of study participants were male (77%), with a mean age of 67 years; 45% of them were diabetic. Individuals with eGFR <30 ml/min/1.73 m², symptomatic hypotension or SBP <95 mmHg and type 1 Diabetes Mellitus were excluded. Dapagliflozin significantly reduced (26% 0.74, CI 0.65 – 0.85) the primary composite outcome of cardiovascular death, hospitalization for HF and emergency visits for heart failure, as well as improved HF symptoms, confirming dapagliflozin as a new standard of care for HFrEF in patients with and without T2DM . ( 19)
2.5 EMPEROR-REDUCED Study
Evaluated patients with HF NYHA functional class II-IV and EF ≤ 40% who were randomized to receive empagliflozin 10 mg once daily and placebo, in addition to recommended therapy. The primary outcome was a composite of CV death or hospitalization for worsening HF. The effect of empagliflozin on the primary outcome was consistent in patients regardless of the presence or absence of T2DM. The number of cardiovascular deaths and hospitalizations for HF was lower in the empagliflozin group than in the placebo group (25% 0.75, CI 0.65-0.86). eGFR was slower and patients had a lower risk of serious renal outcomes, reducing the risk of progression of kidney disease, including the occurrence of renal death, need for dialysis or transplant. ( 20)
2.6 EMPEROR-PRESERVED Study
This study randomized patients with NYHA functional class II-IV HF and EF> 40% with N-terminal B-type natriuretic peptide (NT proBNP) results> 300 pg per milliliter or> 900 pg per milliliter in patients with atrial fibrillation and separated into a group empagliflozin 10 mg once daily and another placebo, in addition to usual therapy. The primary outcome was a composite of CV death or hospitalizations for HF. In this study, empagliflozin reduced the combined risk of cardiovascular death or hospitalization for HF in patients with HFpEF regardless of the presence or absence of diabetes (21% 0.79, CI 0.69-0.90). It is worth noting that this reduction was almost exclusively due to the reduction in hospitalization for heart failure. The secondary outcome observed a reduction in hospitalization for all causes and in the decline in eGFR. ( 20,21)
2.7 DELIVER Study
This was a randomized, controlled study that evaluated patients with HF EF > 40% with evidence of structural heart disease and elevated NT proBNP, and divided them into two groups, one group received dapagliflozin 10 mg once daily and the other group received placebo, in addition to usual therapy. The primary outcome was a composite of worsening HF (unplanned hospitalization or urgent emergency room visit due to HF decompensation) or CV death. The results showed that dapagliflozin reduced (18% 0.82, CI 0.73 – 0.92) the combined risk of worsening HF or CV death in patients with HFrEF or HFpEF. This finding suggests that the benefits of ISGLT2 may extend to all patients with HF regardless of LVEF . ( 22)
2.8 Recommendation of the Guidelines of the Brazilian Society of Cardiology
The Heart Failure Guideline of the Brazilian Society of Cardiology (SBC) predicts a class I recommendation, level of evidence A, for the use of SGLT2 inhibitors (dapagliflozin or empagliflozin) in symptomatic patients with HFrEF, diabetic or not, already receiving the maximum tolerated optimized dose of beta-blocker, aldosterone antagonist, ACEI, ARB or NRA to reduce cardiovascular outcomes and progression of renal dysfunction, regardless of the initial diabetes status. ( 23)
The 2022 American Heart Association (AHA) Heart Failure Guideline includes SGLT2 inhibitors as the 4th fundamental class in the treatment of patients with HFrEF. The recommendations for use are: HFrEF class I, level of evidence A, and can be introduced from the beginning of treatment; HFpEF class II, level of evidence A; HFpEF class II, level of evidence A. ( 23)
The 2021 European Society of Cardiology (ESC) Heart Failure Guideline provides a class I, level of evidence A recommendation for the use of ISGLT2 (dapagliflozin or empagliflozin) in patients with HF regardless of ejection fraction, to reduce the risk of hospitalization for HF or CV death. The drug is administered daily as a single dose (dapagliflozin and empagliflozin) without the need for dose titration and routine laboratory monitoring. Experts justify the initiation of ISGLT2 in patients hospitalized for heart failure as part of an aggressive approach to rapid and simultaneous optimization of medical therapy . ( 23,24)
The United States Food and Drug Administration (FDA) has expanded the indication for dapagliflozin for the treatment of HF regardless of ejection fraction to include HFmrEF and HFpEF. ISGLT2 was previously approved in the US for adults with HFrEF. The expanded indication is based on data from the DELIVER trial, which demonstrated clear clinical benefit of ISGLT2 in patients with heart failure regardless of left ventricular function. ( 24)
Given the relevance of the topic and the high prevalence of HF among the population, the present study aimed to evaluate the frequency of use of Sodium Glucose Cotransporter Type 2 Inhibitors (SGLT2i) in patients with heart failure in the outpatient clinics of the Hospital do Servidor Público Municipal (HSPM-SP), since their use is already established and recommended by cardiology societies in Brazil and worldwide.
3. METHODOLOGY
This was an observational, descriptive and cross-sectional study, carried out at the Hospital do Servidor Público Municipal de São Paulo (HSPM) between November 2023 and April 2024.
Sixty-five patients with heart failure documented by echocardiogram and electronic medical records were included, of both sexes, over 18 years of age who agreed to participate in the study through the Free and Informed Consent Form – TCLE (ANNEX A), either voluntarily or with the acceptance of a guardian.
Exclusion criteria were: age under 18 years; patients without criteria for HF by echocardiogram or who did not have such diagnosis recorded in their medical records; lack of consent or signature of the TCLE.
According to Montera et al., the diagnostic criteria for HF considered according to the Brazilian Society of Cardiology were:
- Major criteria: report in the medical record of the occurrence of pulmonary rales; paroxysmal nocturnal dyspnea; acute pulmonary edema; pathological jugular distension; hepatojugular reflux; central venous pressure > 16 cmH2O; presence of cardiomegaly on chest radiograph, presence of third heart sound (gallop); weight loss > 4.5 kg in 5 days in response to treatment ;
- Minor criteria: report in medical records of dyspnea on exertion; nocturnal cough; pleural effusion; malleolar edema; hepatomegaly; tachycardia (HR > 120 bpm); functional capacity 1/3 of the maximum previously recorded.
A patient was considered to have heart failure if he or she met 2 major criteria; 5 minor criteria; or 1 major and 2 minor criteria of the above.
For analysis purposes, the classification was also made according to the ejection fraction presented, with the aim of distinguishing whether the prescription of iSGLT2 was being made based on this criterion (reduced EF) or in the same way for all patients with HF in light of the new studies.
To this end, the following criteria were considered according to Fernandes-Silva et al. (2020):
- Heart failure with preserved ejection fraction (HFpEF): for patients with LVEF > 50% on echocardiogram and/or elevated BNP and NT-pro-BNP; Structural alteration and/or diastolic dysfunction;
- Heart failure with intermediate ejection fraction (HFmrEF): for patients with LVEF 40 – 49% on echocardiogram and/or elevated BNP and NT-pro-BNP; Structural alteration and/or diastolic dysfunction;
- Heart failure with reduced ejection fraction (HFrEF): for patients with LVEF < 40% on echocardiogram and/or elevated BNP and NT-pro-BNP; Structural alteration and/or systolic dysfunction.
Patients were randomly included based on echocardiogram exams from the last 30 days selected by the researcher at the Fundação Instituto de Pesquisa e Estudo de Diagnóstico por Imagem (FIDI), a program used by HSPM to access imaging exams routinely requested in all outpatient clinics.
Based on the patient’s name, the registration form was identified in the electronic medical record (SGH – Hospital Management System software), with the patient’s telephone contact and an invitation was made to voluntarily participate in the work.
If accepted, the informed consent form was sent for signature and a brief interview was conducted to ask whether the patient was taking the medication “Dapagliflozin” among his/her regular medications. If the answer was positive, the specialist who prescribed it was asked; this information was later confirmed with the HSPM pharmacy, which was responsible for filling the high-cost prescription (when applicable) or checking the electronic medical record.
If the patient’s response regarding the use of iSGLT2 was negative, the patient was asked what the reason(s) were, as shown in Table 1 below:
Table 1 – Table of patient responses to refusal to use iSGLT2.
( ) “My doctor never prescribed it.” ( ) “I didn’t have the financial means to buy it, even with the discount from Farmácia Popular.” ( ) “I was unable to access the high cost (due to distance, mobility difficulties or other reasons)”. ( ) “I was afraid to use it because of adverse effects or drug interactions.” ( ) “I had side effects and stopped using it.” ( ) “I didn’t want to use it because I was already taking a lot of medications (Prior polypharmacy)”. ( ) “Another reason.”
Source: The authors (2024).
In addition to the questions presented, patients were asked about their age, gender and presence of associated diabetes to gain a broader understanding of the sample profile.
The data obtained were entered into Microsoft Excel 2017 for subsequent statistical evaluation and creation of graphs and tables. No personal data or data that could identify the patients were revealed. Continuous variables were presented as means ± standard deviations of the means and categorical variables were represented as absolute frequencies and percentages.
This research complied with the ethical aspects recommended by CNS Resolution 466/2012, in art. III, which implied respect for the research participant’s dignity and autonomy, recognizing their vulnerability, ensuring their willingness to contribute and remain, or not, in the research, through the Free and Informed Consent Form (CFM, 2012), as well as Resolution 1931/2009 CFM, Chapter XII, which deals with medical teaching and research. In this sense, the responsible researcher declared in the commitment form that he knows and complies with the Brazilian Ethical Resolutions.
The project aimed to fully respect the dignity of the human being and during the application of the questionnaire both the patient and his/her family were welcomed, and any doubts and questions about the project were clarified. Questions that could evoke embarrassment or negative feelings related to the patient’s socioeconomic status were avoided.
The data were evaluated only by the main researcher and statistician, and no personal data or data that could identify the patients were revealed.
Regarding ethical aspects, this study followed the guidelines established by Resolution 196/1996 of the National Health Council, which advocated respect for the dignity and autonomy of participants, recognizing their vulnerability and guaranteeing their willingness or not to participate in the research through the Free and Informed Consent Form. In addition, the study was submitted for approval by the HSPM research ethics committee, under opinion number 642.300/CAAE 76590123400005442, through the Brazil Platform, in accordance with resolution 196/1996 of the National Health Council, which regulates research involving human beings.
4. RESULTS
Table 2 presented the sample profile, highlighting the participants’ gender, age groups, the number of diabetics using or not using dapagliflozin and the mean ejection fractions.
Of the 65 patients included in the study, 46% were male and 54% were female. Ages ranged from 40 to 91 years, with an overall mean of 74.4 ± 10.0 years. In addition, 38 patients (58.5%) were diabetic, and of these, only 11 (28.9%) used dapagliflozin. Of those who did not use the drug, 37% were men between 60 and 89 years old and 55.6% were women between 60 and 89 years old. Most patients had preserved ejection fraction.
Table 2 – Profile of patients with heart failure treated at the Hospital do Servidor Público Municipal de São Paulo, from November 2023 to April 2024, São Paulo – SP.
Gender Age Diabetics using dapagliflozin (n=11) Diabetics who do not use dapagliflozin (n=27) Mean ejection fraction Masculine 40-49 years old 0 (0.0) 0 (0.0) – 50-59 years old 0 (0.0) 1 (3.7) 72% 60-69 years old 0 (0.0) 3 (11.1) 53% 70-79 years old 2 (18.2) 3 (11.1) 57% 80-89 years old 3 (27.3) 4 (14.8) 52% 90 years or older 0 (0.0) 0 (0.0) – Feminine 40-49 years old 0 (0.0) 1 (3.7) 68% 50-59 years old 0 (0.0) 0 (0.0) – 60-69 years old 2 (18.2) 3 (11.1) 58% 70-79 years old 2 (18.2) 6 (22.2) 57% 80-89 years old 2 (18.2) 6 (22.2) 59% 90 years or older 0 (0.0) 0 (0.0) –
Frequencies are represented as n (%). Percentages are relative to the total column. Ejection fraction is represented as a mean percentage within the respective sex and age stratum.
Source: The authors (2024).
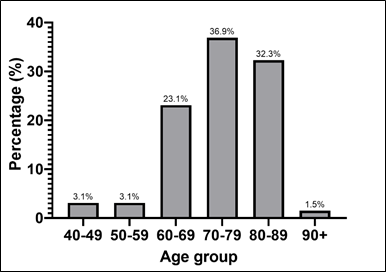
Patients aged 70-89 years (36.9%) constituted the largest portion of the sample. The age groups of 80-89 years and 60-69 years also presented a significant number of patients, with 32.3% and 23.1% respectively. On the other hand, the age groups of 40-59 years were the least represented, with only 3.1% ( Graph 1).
Graph 1 – Patients with HF and average age.
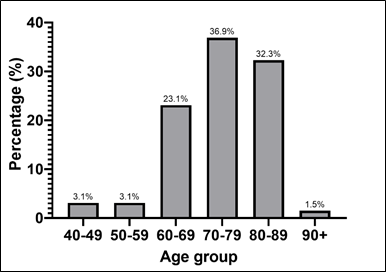
Source: The authors (2024).
Regarding the gender characteristics of patients diagnosed with heart failure, 53.8% were female and 46.2% were male (Graph 2).
Graph 2 – Gender of patients with HF.
Source: The authors (2024).
Among the patients in the analyzed group, it was observed that only 23.1% of patients with HF used dapagliflozin, while 76.9% did not use the medication (Graph 3).
Graph 3 – Patients with HF who do or do not use dapagliflozin.
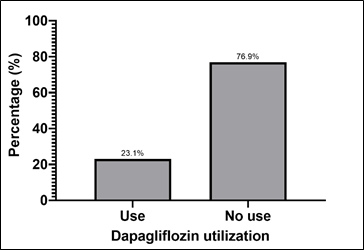
Source : The authors (2024).
Patients with HF who did not use dapagliflozin had different reasons for not using the drug. Among the 50 patients (76.9%) in the study who were not on optimized therapy, the main reason given for not using dapagliflozin was the lack of a prescription for the drug by their physician (74% – Graph 4).
Graph 4 – Patients with IC who do not use of dapagliflozin and their reasons for not using the medication.
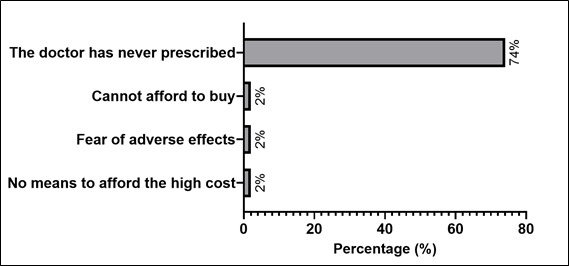
Percentages are relative to patients who did not use dapagliflozin (n=50).
Source: The authors (2024).
Among the 15 patients who used the medication, cardiology and endocrinology (both with 33%) were the specialties that prescribed the medication the most, followed by geriatrics. Nephrology and internal medicine were also present, but with little representation ( Graph 5).
Graph 5- Patients with HF who are using dapagliflozin and the respective medical specialties that prescribe the medication.
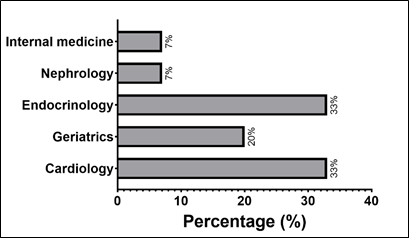
Percentages are relative to patients using dapagliflozin (n=15).
Source: The authors (2024).
Overall, the mean ejection fraction of the sample of 65 patients was 56.5% ± 13.3%. Among patients who used dapagliflozin, this mean was 52.6% ± 14.6%. Among those who did not use the drug, this mean was 57.6% ± 12.8% (Graph 6).
Graph 6- Overall ejection fraction and according to the use or not of dapagliflozin.
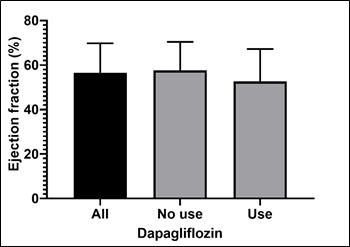
The bars represent the means and the error lines represent the standard deviation.
Source: The authors (2024).
5. DISCUSSION
The data collected showed a slightly higher proportion of women (53.8%) and a large percentage of elderly people (92.3% of participants). This could be explained in part by the greater life expectancy of women and also by the greater demand for health services among women, when comparing the two genders . (24)
Although the participants were randomly selected based on imaging tests performed at the hospital during the study period, a significant number of elderly individuals were observed, which can be explained by the greater number of comorbidities presented in this age group, including a higher prevalence of heart failure, corroborating the literature data. (25)
Although the vast majority of patients were elderly, geriatrics was not the clinic that prescribed Dapagliflozin the most. This may be because patients were already being monitored simultaneously by other specialties such as cardiology and endocrinology, which prescribed the medication before. (26)
Because they were elderly and had multimorbidities, they were probably also taking multiple medications and the addition of another drug may have influenced their therapeutic decision. (26)
The occurrence or not of dementia and frailty, which are usually high in the age group studied, was not investigated in the population studied, which would eventually imply the deprescription of some medications due to individualization of treatment in these cases to the detriment of a rigorous search for controls usually recommended by the main medical societies for younger patients. (28)
On the other hand, among the 76.9% of the research participants who were not on optimized therapy, the main reason reported was the lack of a medical prescription, which is curious in light of the benefits proven in the research presented and the ease of access, since the Brazilian government already makes the medication available in popular pharmacies at a discount and in high-cost pharmacies 100% free of charge. (29)
Also noteworthy is the percentage of patients who did not take the medication for fear of adverse events (2%). Were their respective doctors unable to clarify and address their concerns during consultations? Did the patients not feel comfortable asking questions? These questions call into question the quality of our consultations and the fragile doctor-patient relationship. (30, 31)
Among the clinics that prescribe the medication the most are those specializing in cardiology, endocrinology and geriatrics. (26) These results raise questions about whether knowledge about the benefits of the drug is more widely discussed in the scientific circles of these specialties, leaving out general practitioners, who are just as important as others in caring for the general population, often providing the first care for the individual in the public health system. (32, 33)
The high incidence of Dapagliflozin prescription among endocrinologists and cardiologists was also highlighted. Perhaps a plausible explanation is the valorization of the Cardiovascular Continuum that has been so much debated in the scientific community, especially among specialists in these areas. (34)
6. CONCLUSION
According to the bibliographic literature used in this research, sodium-glucose cotransporter 2 inhibitors have demonstrated several benefits such as nephroprotection, reversal of cardiac modeling, optimization of myocardial contractility, and mainly improvement in the quality of life and prognosis of patients, as well as reduction in hospital readmissions.
A significant percentage of patients in this study with established heart failure were not using the medication, and the most prevalent reason for the absence of optimized therapy was non-prescription (74%). Therefore, it is suggested that more training programs be implemented for physicians in general, both specialists and general practitioners, in order to expand their knowledge on the subject and feel comfortable prescribing dapagliflozin, since it is available through the Unified Health System. In addition, raising awareness on the subject among health professionals is of utmost importance, given the high mortality and morbidity rates in this group of patients. And although the second reason (fear of adverse effects) for patients not using the medication was low in percentage (2%), it is still worth reflecting on the bond that is being created with the users of these services. Therefore, it is necessary to create a respectful atmosphere, in which the patient does not feel embarrassed to ask questions and can return in a timely manner if adverse reactions occur, so that the best possible doctor-patient relationship can be built, with the best therapy for their case.
REFERENCES
- Anker SD, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. For the EMPEROR-Preserved Trial Investigators. The New England Journal of Medicine. 2021;385(16):1451-1461.
- Bochi EA, et al. Emerging Topics in Heart Failure: sodium-glucose cotransporter 2 inhibitors (SGLT2i) in HF. Brazilian Archives of Cardiology. 2021; 116(2):355-358.
- Federal Council of Medicine. CFM 2012. Resolution 1931/2009: Approves the Code of Medical Ethics. Federal Council of Medicine, DOU September 24, 2009, Section I, p. 90 [Accessed on May 5, 2024]. Available at: https://portal.cfm.org.br/etica-medica/codigo-2010/resolucao-cfm-no-1931-2009/ .
- Brazil. Ministry of Health. National Health Council. 1996. Resolution 196/1996: Approves the guidelines and regulatory standards for research involving human beings. CNS; 1996 [Accessed on May 5, 2024]. Available at: https://conselho.saude.gov.br/resolucoes/reso_96.htm .
- Dewan P, et al . Efficacy and safety of sodium-glucose cotransporter 2 inhibition according to left ventricular ejection fraction in DAPA-HF. Eur J Heart Fail. 2020; 22(7):1247-1258.
- Ejiri K, et al . Effect of Luseogliflozin on Heart Failure with Preserved Ejection Fraction in Patients with Diabetes Mellitus. J Am Heart Assoc. 2020;9(16):e015103.
- Fernandes-Silva MM, et al. Emerging Topics in Heart Failure: Heart Failure With Preserved and Mid-Range Ejection Fraction. Brazilian Cardiology Archives. 2020; 115(5):949-952.
- Heerspink HJL, et al. Dapagliflozin in Patients with Chronic Kidney Disease. For the DAPA-CKD Trial Committees and Investigators. The New England Journal of Medicine. 2020; 383(15):1436-1446.
- Mcdonagh TA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA ) of the ESC. European Heart Journal. 2021; 42(36):3599-726.
- McMurray JJV, et al. Dapagliflozin in Patients with Heart Failure and . Reduced Ejection Fraction. For the DAPA-HF Trial Committees and Investigators. The New England Journal of Medicine. 2019; 381(21):1995-2008.
- Mann DL, et al. Braunw ald’s heart disease: a textbook of cardiovascular medicine. 10 ed. Philadelphia: Elsevier; 2015.
- Martins CNG, et al. Possible Mechanisms of Action of SGLT2 Inhibitors in Heart Failure. ABC: Heart Failure & Cardiomyopathy. 2021; 1(1):33-43.
- Miranda JSS, et al. I Need Help: how to identify patients with advanced cardiac dysfunction? ABC: Heart Failure & Cardiomyopathy. 2022; 2(2):157-64.
- Montera MW. II Brazilian Guideline for Acute Heart Failure. Brazilian Society of Cardiology (SBC). Brazilian Archives of Cardiology. 2009; 93(3):1-65.
- Neal B, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. For the CANVAS Program Collaborative Group. The New England Journal of Medicine,. 2017; 377:644-657.
- Packer M, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. For the EMPEROR – REDUCED Trial Committees and Investigators. The New England Journal of Medicine. 2020; 383(15):1413-1424.
- Ní Mhaolá in AM, Gallagher D, O’Connell H, Chin AV, Bruce I, Hamilton F. Subjective well-being amongst community-dwelling elders: what determines satisfaction with life? Findings from the Dublin Healthy Aging Study Int Psychogeriatr. 2012;24(2):316-23.
- Packer M, et al. Design of a prospective patient-level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure. European Journal of Heart Failure. 2020; 22(12):2393-2398.
- Solomon SD, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. For the DELIVER Trial Committees and Investigators. The New England Journal of Medicine. 2022;387(12):1089-1098,
- Talha KM, Anker SD, Butler J. SGLT-2 Inhibitors in Heart Failure: A Review of Current Evidence. International Journal of Heart Failure. 2023;5(2):82-90.
- Zinman D, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. For the EMPA REG investigators. The New England Journal of Medicine. 2015;373:2117-2128.
- McMurray JJV, et al . Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381(21):1995-2008.
- Bocchi EA, Biolo A, Moura LZ, Figueiredo Neto JA, Montenegro CEL, Albuquerque DC. Emerging Topics in Heart Failure: Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) in HF. Arq. Bras. Cardiol. 2021;116(2) :355-8.
- Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020 Sep 19;396(10254):819-829.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383(15):1413-1424.
- Costa C, Costa V, Alves C, Santos B, Barbosa Junior F, Areda C, Karnikowski M, Oliveira R. Access to and sources of obtaining medicines used by the elderly in an educational program in the Federal District. OBSERVATORY OF THE LATIN AMERICAN ECONOMY. Vol. , 22.DOI:10.55905/oelv22n9-102. 2024.
- Prado MAMB, Francisco PMSB, Barros MBA. Diabetes in the elderly: use of medications and risk of drug interactions. Ciência & Saúde Coletiva, 21(11), 3447–3458. doi:10.1590/1413-812320152111.2446201. 2016.
- Butt JH, Jhund PS, Belohlávek J, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients With Heart Failure: A Prespecified Analysis of the DELIVER Trial. American Heart Association. DOI: 10.1161/CIRCULATIONAHA.122.061754. 2022.
- Ministry of Health. Report on Recommendation of Clinical Protocol and Therapeutic Guidelines for Type 2 Diabetes Mellitus No. 882. https://www.gov.br/conitec/pt-br. 2024 .
- Fioretto P, Mansfield TA, Ptaszynska A, Yavin Y, Johnsson E, Parikh S. Long-Term Safety of Dapagliflozin in Older Patients with Type 2 Diabetes Mellitus: A Pooled Analysis of Phase IIb/III Studies. Drugs Aging. Jul;33(7):511-22. doi: 10.1007/s40266-016-0382-1. 2016.
- Ratanawongsa N, Karter AJ, Parker MM, Lyles CR, Heisler M, Moffet HH, Adler N, Warton EM, Schillinger D. Communication and medication refill adherence: the Diabetes Study of Northern California. JAMA Intern Med.Feb 11;173(3):210-8. doi: 10.1001/jamainternmed.2013.1216. 2013.
- Blair HA. Dapagliflozin: A review in symptomatic heart failure with reduced ejection fraction. Am J Cardiovasc Drugs. 2021 Nov;21(6):701-710. doi: 10.1007/s40256-021-00503-8. 2021.
- Slomski A. Benefit of Dapagliflozin When Used With Cardiovascular Medications in Patients With Type 2 Diabetes. JAMA. 328(9):817. doi:10.1001/jama.2022.14696. 2022.
- Seidu S, Alabraba V, Davies S. et al. SGLT2 Inhibitors – The New Standard of Care for Cardiovascular, Renal and Metabolic Protection in Type 2 Diabetes: A Narrative Review. Diabetes Ther 15, 1099–1124. https://doi.org/10.1007/s13300-024-01550-5. 2024.
