REGISTRO DOI:10.5281/zenodo.10440601
Ícaro José Araújo de Souza
Larissa Dacier Lobato Comesanha
Isabel Campos Lobato Khaled
Karem Miléo Felício
João Soares Felício
Natércia Neves Marques de Queiroz
Abstract
Scientific researches have reinforced the importance of Vitamin D (VD) supplementation in the pathogenesis of diabetes and glycemic control through the active form of VD and insulin sensitivity and secretion. In our study, we conducted a systematic review to evaluate the effects of oral vitamin D supplementation on glycemic control in type 1 diabetic patients through glycated hemoglobin levels. We searched PubMed (MEDLINE) and Cochrane Library databases. A total of 10 studies were included in our study, with an average age between 6 and 44 years old. The intervention duration ranged from 3 to 18 months. Half of the studies showed improvement in glycemic profile, adopting HbA1c as an observable variable and 5 did not show any change. The improvement group had a baseline VD mostly with deficiency values. We found a effect through glycated hemoglobin especially with a daily supplementation with cholecalciferol within less than 6 months. However, the articles that showed better regulation in glycemic parameters had a previous deficient baseline VD. In addition, the studies analyzed were not conclusive regarding genetic polymorphisms that predispose impaired glycemic control associated with hypovitaminosis, probably due to differences in ethnicity and exposure to ultraviolet radiation.
Keywords: Diabetes Mellitus type 1, Vitamin D, HbA1c, Glycemic control, Systematic Review.
BACKGROUND
Type 1 diabetes mellitus (DM1) has a relevant epidemiological impact, with an annual growth of approximately 3% and a global figure of 1,211,900 carriers of this endocrinopathy (LOVIC et al., 2020; MAGLIANO; BOYKO, 2021). In addition, recent studies have shown that the annual incidence of this disease is increasing, with a value close to 2.02~5.3 per 100,000, establishing a worrying pattern for health authorities, as reports indicate that in 2021 USD 966 billion were spent on diabetes-related health expenditure (MAGLIANO; BOYKO, 2021; WANG et al., 2022). By 2040, the number of people with DM1 is projected to reach 13.5-17.4 million, reinforcing the severity of the disease and its impact on global mortality (OGROTIS; KOUFAKIS; KOTSA, 2023). According to the IDF, approximately 6.7 million individuals are estimated to have died as a result of diabetes or its complications (MAGLIANO; BOYKO, 2021).
Vitamin D (VD) corresponds to an organic substance, which studies suggest a preventive role in chronic non-skeletal diseases (AL REFAIE et al., 2022; ZHANG et al., 2019). Although primarily associated with use in bone metabolism, numerous scientific researches have focused on VD supplementation in diabetic patients (GRAMMATIKI; KARRAS; KOTSA, 2019). Recent hypotheses reinforce the importance of supplementation in the pathogenesis of diabetes and glycemic control through a positive correlation between the active form of VD and insulin sensitivity and secretion (NAJJAR et al., 2021; SACERDOTE et al., 2019). Evidence from an observational study has shown that supplementation decreases the risk of developing T1DM and, in that case, this micronutrient shows a biological potential role in the glycemic profile of individuals diagnosed with diabetes (LI et al., 2018; MANOUSAKI et al., 2021).
Relevant actions of VD are related to immunomodulatory function in diabetes through the transcription of the vitamin D receptor (VDR), which is responsible for increasing insulin sensitivity (CALMARZA et al., 2022; GRECO; LENZI; MIGLIACCIO, 2019). The literature addresses possible participation in the pathogenesis of extraskeletal diseases through the inhibition of autoimmune and inflammatory responses (HE et al., 2022a; MARETZKE et al., 2020). It is also observed that adequate levels of VD can provide a decrease in pro-inflammatory cytokines involved in the pathogenesis of TD1M, while its deficiency is associated with insulin resistance, which favors the process of disease (AHMED et al., 2019; ETTEN; MATHIEU, 2005).
The mechanism by which VD modulates glycemic homeostasis involves alterations in gene expression that are responsible for cell death and beta-cell function (ALTIERI et al., 2017). Moreover, some experimental animal studies demonstrate that VD can prevent pancreatic islet cell death and restore insulin secretion (PANJIYAR et al., 2018; WOLDEN-KIRK et al., 2014). There is still no clear relationship between VD levels and HbA1c in the literature, because some studies reported a beneficial association in individuals with established T1DM, while other authors suggested that there was no correlation between VD levels and HbA1c values in newly diagnosed children or in patients with a relatively compensated T1DM (LIU et al., 2018; POVALIAEVA et al., 2020). This review aimed to evaluate the effects of oral vitamin D supplementation on glycemic control in type 1 diabetic patients through glycated hemoglobin levels.
METHODS
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement, as evidenced in Figure 1.
- Selection of studies
Studies were considered eligible if they (a) were randomized controlled trials (b) evaluated/approached the impact of VD supplementation on HbA1c in TDM1 patients; (c) used oral VD formulations containing cholecalciferol OR ergocalciferol OR alphacalcidol; (d) had a trial length > 2 months; (e) trials with an intervention-control group or intra individual analysis.
Exclusion criteria were as follows: (a) intramuscular delivery of VD with different absorption routes other than oral; (b) studies limited to the impacts of VD deficiency; (c) studies involving participants with type 2 diabetes (T2DM), gestational diabetes or systemic conditions that could alter VD metabolism, patients at risk of developing T1DM, or non-T1DM patients; (d) combination therapy for VD administration; (e) ongoing studies with unpublished final results; (f) observational studies, case reports, editorials, commentaries and poster abstracts.
The strategic PICOS (Population, Intervention, Comparison, Outcome, Study design) was also used in the selection of studies for greater specificity (Table 1). We selected studies carried out with type 1 diabetics who underwent vitamin D supplementation. Results of the glycemic profile were analyzed and only randomized clinical trials were used.
- Data sources and search strategies
We searched databases including PubMed (MEDLINE) and Cochrane Central Register of Controlled Trials (CENTRAL) from inception to April 2023 to identify studies published in English, Portuguese or Spanish. The final search was performed on April 13, 2023.
The following search strategy was used in PubMed and tailored to each database when necessary: (randomized controlled trial OR controlled clinical trial OR random OR clinical trial OR controlled trial OR RCT NOT review NOT animal) AND (vitamin D OR vitamin D2 OR vitamin D3 OR cholecalciferol OR ergocalciferol OR alphacalcidol OR alfacalcidol OR paricalcitol OR doxercalciferol OR calcitriol OR 25-Hydroxyvitamin D) AND (Diabetes OR Glycemic Control OR Glucose OR Hyperglycemia OR HbA1c OR Glycated Hemoglobin OR Diabetes Mellitus Type 1 OR Type 1 Diabetes Mellitus OR Diabetes Mellitus, Insulin Dependent, 1 OR Insulin-Dependent Diabetes Mellitus 1OR Diabetes Mellitus Juvenile Onset OR IDDM OR T1DM OR Insulin resistance OR Insulin sensitivity). The finding searches can be seen in table 2.
- Data extraction
Pairs of independent reviewers screened the titles and abstracts of each study according to eligibility criteria before completing the initial screening via EPPI Reviewer software. Any conflicting studies were subjected to a full-text evaluation, and if discrepancies in decision remained, a third reviewer stepped in to arbitrate.
For all included trials, two reviewers independently extracted information regarding the characteristics of the studies (year of publication, authors, city and sample size), the participants (gender, age, body mass index (BMI)), the intervention performed (treatment, type, dose, therapy duration and administration route), glycemic control (pre and post-test values of HbA1c and fasting glucose) and serum VD.
D) Risk of bias
The quality of each study was independently assessed according to the Cochrane Risk of Bias Tool (RoB2) by two reviewers. Five domains of bias (i.e., randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results) were evaluated and reported. The Cochrane Handbook for Systematic Reviews of Interventions was used as a reference guide during the evaluation. A judgment of “high” indicated a high risk of bias, “low” indicated a low risk of bias, and “some concerns” indicated the presence of bias due to lack of information or uncertainty about the potential for bias. Thus, the studies were categorized as having low or high risk of bias or some concerns (HIGGINS, 2008). The risk of bias was assessed by two authors independently. Any discrepancy in the assessment of RoB2 was discussed to attain a consensus.
Regarding the methodological quality of the studies included in the present review, some authors were not clear about some criteria evaluated. Overall, the data had a low risk of bias and no high risk of bias was identified, as shown in figure 2.
RESULTS
Of all the 2835 articles, 72 remained as potentially eligible after the title and abstract screening. Then, the final screening was performed by reading full articles and the remaining 10 eligible articles for review construction.
Characteristics of all 10 studies are presented in table 3. Among those, 4 studies involved Middle Easterns, 2 were conducted in South America, 2 enrolled European individuals, 1 was developed in North America and 1 was performed in Asia (Figure 3). Those studies involved participants with an average age between 6 and 44 years old. The intervention duration ranged from 3 to 18 months. Vitamin D3 was used in 8 studies, Vitamin D2 in 1 and calcitriol was used in 1 trial. In addition, 4 studies performed a comparison of intra-individual VD supplementation and 6 among a control group.
The selected trials were classified as studies with control groups or intra-individual groups, that is, comparing each other at two points in the study, after and before the intervention, for example. In the sample selected, most of the studies involved were case-control.
Moreover, for discussion and clarification purposes, it was possible to segment the trials between two main characteristics, those who showed improvement in glycemic control, adopting HbA1c as an observable variable in the studies, and those who did not show any change. None of the studies worsened this observable parameter, as visualized in table 4.
With this segmentation, it is possible to observe in more detail the characteristics of the studies of each group, that is, the group improves and the group does not change. At first, the improvement group has a baseline VD mostly with deficiency values (< 20 ng/mL) compared to the group unchanged, in which a tendency to insufficiency is observed (20-29 ng/mL) (HOLICK et al., 2011a). The baseline and follow-up values of VD can be seen in figure 4, however, not all articles provided both data. In addition, there are studies with an average duration of 7.2 months vs. 8.4 months, the improvement group and the no change group, respectively. Other differences can be highlighted, such as VD type, dose, and frequency as shown in table 5.
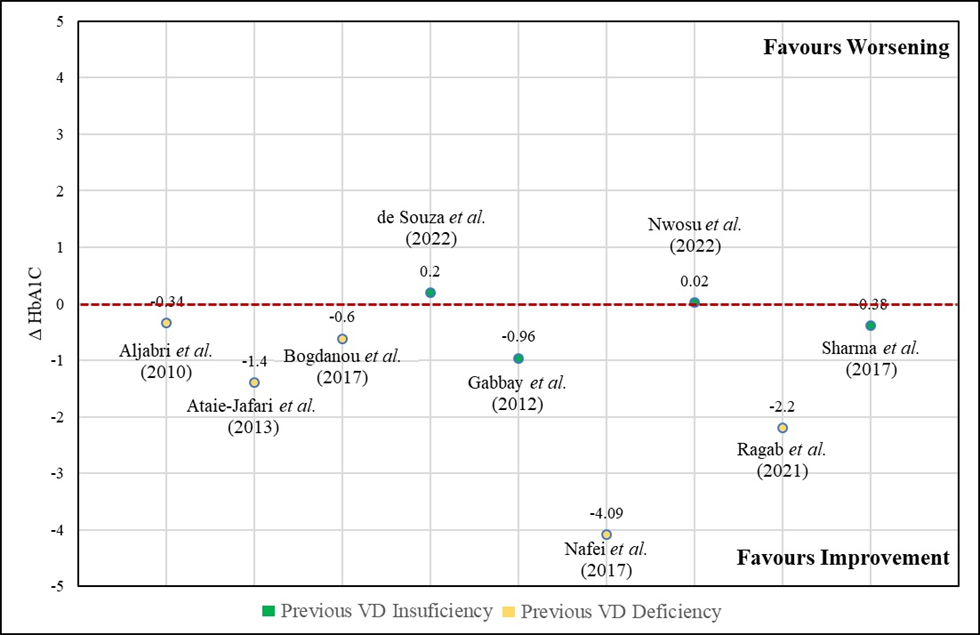
Finally, it was possible to perform a brief analysis to glimpse the difference between studies that had a different baseline vitamin D classification, that is, baseline deficiency vs baseline insufficiency. In the total analysis, a robust effect is identified, with an improvement in HbA1c, varying from +0.2 to -4.09. However, in the individual analysis of the subgroups, it is possible to highlight an extremely robust effect of improvement in the glycated hemoglobin profile only in the group with previous deficiency (variation from -0.34 to -4.09), while the group with insufficiency has variations up to even an increase in glycated hemoglobin, but with a slight tendency to improve HbA1C values (range from 0.2 to -0.96), as highlighted in figure 5.
DISCUSSION
This review showed that VD supplementation did not harm HBA1C in patients with DM1, only improving or not changing this parameter. Retrospective studies confirm such findings through a significant improvement in glycemic and metabolic control after VD supplementation with cholecalciferol due to a decrease in HbA1c levels and, consequently, in the need for insulin (GIRI et al., 2017; SAVASTIO et al., 2016). These factors possibly demonstrate the impact of vitamin D3 in preserving the residual beta cell function of patients with T1DM (INFANTE et al., 2019).
Type of Diabetes
A recent meta-analysis has shown that vitamin D supplementation could be effective at improving glycemic control, especially in HbA1c, in vitamin D deficient (<20ng/ml) or non-obese type 2 diabetes (T2DM) patients, but there was no significant association in the subgroups of vitamin D insufficiency (20-30ng/ml) or sufficiency (>30ng/ml) (WU et al., 2017). Despite T2DM and T1DM had different etiology have different etiologies, the best response found to improve glycemic levels in people with vitamin D deficiency is probably related to its extraskeletal factors that will probably reach optimized responses when there are sufficient levels, so this improvement is seen from the way in people who had deficiency regardless of the type of diabetes.
This same effect may explain why longer trials and higher doses demonstrate a better effect on blood glucose levels. In our sample, the studies with an improvement in glycemic profile ranged from 12 to 72 weeks. There was no clear pattern in research in T2DM and studies such as Harron et al, Musazadeh et al and Kru-Poel showed that there was no significant effect on HbA1c in long-term interventions with a period trial longer than 3 months (KRUL-POEL et al., 2017; MUSAZADEH et al., 2023; NIGIL HAROON et al., 2015). However, Lee et al and Mirhosseini et al demonstrated that longer supplementation could have a larger reduction in glycemic control (LEE et al., 2017; MIRHOSSEINI et al., 2017). T2DM patients received similar doses to obtain non-skeletal benefits of VD, in agreement with the studies described in our sample (MIRHOSSEINI et al., 2017; MUSAZADEH et al., 2023). Nevertheless, there are still conflicting results in the literature due to heterogeneity in the establishment of dose and duration of VD supplementation, as some studies with daily supplementation > 11.200 UI revealed no effects on glycemic control, while a dosage of 4000 IU/day have shown advantages (CHRYSOSTOMOU, 2016; GRAMMATIKI; KARRAS; KOTSA, 2019).
Type of vitamin D
Among the studies, cholecalciferol, ergocalciferol, alfacalcidol and calcitriol were used as VD therapy. In the present study, ergocalciferol showed no advantages in glycemic control of diabetic patients and few investigations on this association with T1DM were found. In literature searches, non–significant results were found when it utilized healthy subjects and type 2 diabetes (T2DM), reinforcing the lack of knowledge about the effect of ergocalciferol in the population of interest (MITCHELL et al., 2015; QI et al., 2022). However, previous meta-analyses that compared the effectiveness of vitamin D2 and D3 supplementation, concluded that cholecalciferol increased vitamin D levels, regardless of dose, mode, or vehicle of administration in comparison to ergocalciferol (BALACHANDAR et al., 2021; TRIPKOVIC et al., 2012).
Despite the low number of articles available with an approach to the benefits of administration of calcitriol supplementation in glycemic control, when we cross our database with other literature we find results that complement each other. Pertinent randomized studies indicate that there is no great influence on therapy with calcitriol, as its results in decreasing the required insulin dose were immediate and brief, without positively affecting glycemic levels, which perhaps generates hypotheses about the existence of an initial abbreviated modulation in the autoimmune process against pancreatic beta cells before the full development of the disease (BIZZARRI et al., 2010; NAPOLI et al., 2013; PITOCCO et al., 2006). These negative findings in calcitriol may be related to a low dose administration, in order to avoid the risk of adverse side effects related to hypercalcemia (INFANTE et al., 2019). However, the use of alfacalcidol, a prodrug of the active form of calcitriol, may preserve B-cells by higher fasting C-peptide levels and lower daily insulin doses in the intervention group (ATAIE-JAFARI et al., 2013).
Dose and frequency of supplementation
It was observed a majority improvement in glycemic profile in those who used cholecalciferol in a daily dosage. Associated with this, the studies that obtained a positive result, that is, an improvement in HbA1C levels are also not cohesive in terms of VD dosage, but the predominance of a daily frequency stands out, which differs them from the group without changes in the glycemic profile. In contrast, previous studies such as Mager et al. state that daily and monthly VD supplementation therapies are equally effective methods in increasing serum 25(OH)D concentrations to target ranges with similar levels of adherence and safety (MAGER et al., 2017). However, daily treatment may have enhanced the immunomodulatory and anti-inflammatory autoimmune insulitis in T1DM (MAEDA et al., 2014). These effects of chronic inflammation could be reduced through an increasing calbindin expression against the apoptosis cells mechanisms and inactivation of inflammatory cytokines associated with insulin resistance (PFEIFER et al., 2001).
The VD doses used in the articles that demonstrated benefits regarding HbA1c showed two patterns, one with lower doses that ranged from 10 and 20UI daily, while the other doses were higher and were concentrated between 2000 and 4000 IU daily. Among the studies that did not improve glycemic profile dosages were administered from 2000 IU to 50.000 IU in a daily, weekly, or monthly pattern. The divergence may be related to the difference in administration time because according to Pfeifer et al., weekly or monthly doses have different biological effects than daily administration and are probably less effective (BERGMAN et al., 2013; CONTRERAS-BOLÍVAR et al., 2021; ROSSINI et al., 2012). In addition, doses administered without a standard pattern could contribute to these different results (QUEIROZ, 2018).
Duration of studies
In the selected sample, studies with 3 months to 18 months duration were observed, and thereafter there is no difference between the groups that showed an improvement or not in HbA1C. Despite this, it is possible that 4 of the 5 studies that presented positivity changes in glycated hemoglobin are short-term studies, that is, within less than 6 months. As mentioned above, a longer analysis may shed more light on the effects and limitations of the intervention.
Gender and VD
A recent huge observational study has pointed to a sex-dependent association possibly caused by a synergistic interaction of estrogens and vitamin D, however, the association is best reported according to the existence of a deficiency that is more prevalent in females (HOLICK et al., 2011b). In our studies this analysis cannot be done, since the trials do not report in their entirety the distribution between genders. Despite this, 2/3 of the studies from which we have data that have improved the parameter of glycated hemoglobin have a prevalence of females and with a status of deficiency before VD supplementation. Associated with the fact that the unchanged group in the aspect of glycated hemoglobin is mostly male. Furthermore, it is possible to see in our analysis that previously deficient individuals had a better effect on glycated hemoglobin after supplementation. Thus, it is possible that people enrolled as type 1 diabetic individuals foster the findings of a sex-dependent association, although other characteristics must be taken into account.
VD deficiency and diabetes
According to our findings in people with T1DM, the unchanged group had a tendency to insufficiency VD baseline and the improvement group had a baseline VD mostly with deficiency values. A deficient vitamin D value plays an important risk factor in the onset and development of insulin resistance (IR) through a modulation of depolarization-induced insulin secretion via intracellular Ca2+ level regulation in pancreatic β-cells (CHEN et al., 2021; SZYMCZAK-PAJOR; DRZEWOSKI; ŚLIWIŃSKA, 2020). The baseline samples of the studies highlight that most of the population of the trials has VD insufficiency, even though not all of them had a deficiency (<20ng/ml), almost all were at least in a level of insufficiency (21-29ng/ml) (SOOY et al., 1999).
The literature addresses that Vitamin D deficiency may contribute to the onset and worsening prognosis of diabetes (SCRAGG; SOWERS; BELL, 2004; SEGOVIA-ORTÍ et al., 2020; WIMALAWANSA, 2018). In the case of T1D, autoimmunity seems to be a major factor in inducing this process in β-cells death. The deficiency in VD levels may contribute to the onset of this immune response through the weakening of the inhibitory effect on T cells, triggering the release of chemokines that attack β-cells (FU; R GILBERT; LIU, 2013; LITTORIN et al., 2006; TAKIISHI et al., 2010). Vitamin D acts to reduce the inflammatory mechanisms responsible for T1DM (HE et al., 2022b; HYPPÖNEN et al., 2001).
In summary, the literature states that VD influences epigenetic changes and characteristics in the development of diabetes at their level and in cellular processes. In this way, the deficient VD could, in their decline, promote the onset of diseases such as diabetes (BERRIDGE, 2017). Our revision corroborated this hypothesis, with studies that showed more prevalent VD deficiency had a major improvement in glycemic control, this affirmation should be confirmed and also stimulated questions about the importance and effectiveness of oral VD supplementation.
Genetic Aspect
Vitamin D related to genetic polymorphism may predispose to impaired glycemic control associated with hypovitaminosis. Epidemiological studies have shown an association between low serum VD concentration and an increased risk for metabolic syndrome and diabetes (LIPS et al., 2017). This is supported by the finding that polymorphisms of VDR have been linked to diabetes, especially to insulin secretion capacity in humans (OGUNKOLADE et al., 2002; SENTINELLI et al., 2016).
The VDR gene has been investigated as a candidate gene in DM development, impacting susceptibility to T1DM and its complications. Data suggest that VDR genomic linkage positions are highly expressed near genes involved with T1DM risk (RAMAGOPALAN et al., 2010). Furthermore, the prevalence of certain VDR gene polymorphisms has been associated with T1DM susceptibility in populations from Uruguay (FokI (C/T rs2228570)), southern Croatia (Tru9I (G/A rs757343)), Japan (BsmI (A/G rs1544410)), Greece FokI, BsmI, ApaI (C/T rs7975232) and TaqI (T/C rs731236)), Germany and India (BsmI) (THRAILKILL; FOWLKES, 2013).
The studies analyzed are not conclusive regarding genetic aspects. Still, it is worth mentioning the findings in ethnic differences in the populations analyzed, as well as differences in the exposure to ultraviolet radiation in different regions.
Vit D and sun exposure
According to the database regarding the baseline VD of the participants, it was found that the results associated with improved levels of HbA1c are linked to countries in the Middle East, Africa, Western Europe, and Southwest Asia, revealing significant geographic heterogeneity in terms of sun exposure during the research period. Despite the discrepancy between countries, a case-control study demonstrated that regardless of the local exposure to sun, children with T1DM have lower levels of VD, corroborating with the studies used in the present study that showed VD deficiency (GREER et al., 2013).
Scientific evidence in a Brazilian study suggests an important role of the VDR in susceptibility to T1DM, without necessarily being related to the age of onset of the disease (DE AZEVÊDO SILVA et al., 2013). Supporting these findings, a study in several countries such as Egypt, Kuwaiti, Iran, Saudi Arabia, and Korea indicates the same pattern (ABD-ALLAH et al., 2014; CHEON et al., 2015; MOHAMMADNEJAD et al., 2012; RASOUL et al., 2019). In other populations such as Portugal, the United Kingdom, and the United States this phenomenon was not found, suggesting that different genetic and/or environmental backgrounds may be responsible for these differences (CHEON et al., 2015; LEMOS et al., 2008; NEJENTSEV et al., 2004; PANI et al., 2000).
There is still difficulty in determining a specific pattern of which factors most influence increased serum levels of VD due to numerous possibilities, such as genetic interaction, dietary intake, latitude, climate and seasonal variation, sun-seeking behaviors and clothing habits of individuals from different geographic regions (ABD-ALLAH et al., 2014; ELLIOTT et al., 2010; MILLER et al., 2017). Although in many countries the seasonal variation of 25OHD exists, our study analyzed the effect of the increased VD supplementation, regardless of its absolute value.
Strengths and weaknesses
There are also some strengths and limitations of our study. The merit of our study is the evaluation of the effects of Vitamin D supplementation in type 1 diabetic patients. In addition, our analysis takes into account the most recent years of publications on VD and T1DM, thereby increasing our assessment/knowledge of VD supplementation in HbA1c. We also identified influential factors that could impact the supplementation effects, like ethnicity, baseline vitamin D and gender. These findings might encourage further studies to confirm the influence and mechanisms of these factors and allow the provision of supplementation with appropriate timing and in appropriate subpopulations. Additionally, our limitations were heterogeneity owing to the various lengths, doses, and participants involved in the studies, as well as the use of different types of vitamin D. In addition, studies that allowed medication adjustment during the intervention period could confound the effect of VD. Last, some trials included relatively small sample sizes and short intervention durations.
FINAL CONSIDERATIONS
There was a suggestive beneficial effect of vitamin D supplementation on glycated hemoglobin, despite the heterogeneity of the studies included in this review. This significant effect is highlighted in individuals with prior vitamin D deficiency. Additionally, it is suggested that daily vitamin D supplementation with cholecalciferol use has a better response in controlling the glycemic parameter, but further studies are still needed to clarify the differences and efficacy.
Therefore, the beneficial effects that this vitamin can provide to glycemic control are reported from the studies involved, despite the lack of standardization with regard to the sample, dosage, and duration of vitamin administration. Thus, it becomes evident the need for studies with clinical trials that evaluate, with good quality and methodological focus, directed VD supplementation in T1DM individuals and its effects on the glycemic profile.
DECLARATIONS
1 Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
2 Funding
The authors received no specific funding for this work
ETHICAL RESPONSIBILITIES OF AUTHORS
This manuscript has not been published and is not under consideration for publication in any other journal. All authors approved the manuscript and consent to this submission.
3 Author Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. NNMQ, LDLC and IJAS took part in conception and design of study. LDLC and IJAS were responsible for acquisition of data, while KMF, JSF and NNMQ have done the analysis and interpretation of data. LDLC, IJAS and ICLK have drafted the manuscript together. All authors have revised the manuscript critically and approved the version to be published.
4 Acknowledgments
The authors thank Federal University of Pará, University Hospital João de Barros Barreto, Programa de Pós-Graduação em Atenção e Estudo Clínico no Diabetes for their contribution to the research.
LIST OF ABREVIATURES
1,25 (OH)2D3: 1,25-dihydroxyvitamin D3
BMI: body mass index
CKD: chronic kidney disease
FPG: fasting plasma glucose
HbA1c: glycated hemoglobin
HRQol: healthy-related quality of life
IDAA1c: insulin dose–adjusted A1c;
IU: International Units
PICOS: Population, Intervention, Comparison, Outcome, Study design
PRISMA: Preferred Reporting Items for Systematic Reviews
RBCF: residual β-cell function;
SCP: stimulated C-peptide;
SMD: standardized mean differences
T1DM: Type 1 Diabetes Mellitus
T2DM: Type 2 Diabetes Mellitus
TNF-α: tumor necrosis factor alpha
VD: Vitamin D
VDR: Vitamin D receptor
ABD-ALLAH, S. H. et al. Vitamin D status and vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Egyptian children. Gene, v. 536, n. 2, p. 430–434, fev. 2014.
AHMED, A. E.-A. et al. Vitamin D receptor rs7975232, rs731236 and rs1544410 single nucleotide polymorphisms, and 25-hydroxyvitamin D levels in Egyptian children with type 1 diabetes mellitus: effect of vitamin D co-therapy. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, v. Volume 12, p. 703–716, maio 2019.
AL REFAIE, A. et al. Vitamin D and adrenal gland: Myth or reality? A systematic review. Frontiers in Endocrinology, v. 13, 13 out. 2022.
ALTIERI, B. et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Reviews in Endocrine and Metabolic Disorders, v. 18, n. 3, p. 335–346, 9 set. 2017.
ATAIE-JAFARI, A. et al. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clinical Nutrition, v. 32, n. 6, p. 911–917, dez. 2013.
BALACHANDAR, R. et al. Relative Efficacy of Vitamin D2 and Vitamin D3 in Improving Vitamin D Status: Systematic Review and Meta-Analysis. Nutrients, v. 13, n. 10, p. 3328, 23 set. 2021.
BERGMAN, P. et al. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE, v. 8, n. 6, p. e65835, 19 jun. 2013.
BERRIDGE, M. J. Vitamin D deficiency and diabetes. Biochemical Journal, v. 474, n. 8, p. 1321–1332, 15 abr. 2017.
BIZZARRI, C. et al. No Protective Effect of Calcitriol on β-Cell Function in Recent-Onset Type 1 Diabetes. Diabetes Care, v. 33, n. 9, p. 1962–1963, 1 set. 2010.
CALMARZA, P. et al. Vitamin D concentration in type 1 diabetic children. Association with glycemic control, lipidic and bone metabolism. Nutrición Hospitalaria, 2022.
CHEN, X. et al. Sex-Dependent Association of Vitamin D With Insulin Resistance in Humans. The Journal of Clinical Endocrinology & Metabolism, v. 106, n. 9, p. e3739–e3747, 18 ago. 2021.
CHEON, C. et al. Vitamin D receptor gene polymorphisms and type 1 diabetes mellitus in a Korean population. Pediatrics International, v. 57, n. 5, p. 870–874, 6 out. 2015.
CHRYSOSTOMOU, S. Vitamin D Daily short-term Supplementation does not Affect Glycemic Outcomes of Patients with Type 2 Diabetes. International Journal for Vitamin and Nutrition Research, v. 86, n. 5–6, p. 169–183, out. 2016.
CONTRERAS-BOLÍVAR, V. et al. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients, v. 13, n. 10, p. 3491, 1 out. 2021.
DE AZEVÊDO SILVA, J. et al. Vitamin D receptor (VDR) gene polymorphisms and age onset in type 1 diabetes mellitus. Autoimmunity, v. 46, n. 6, p. 382–387, 30 set. 2013.
ELLIOTT, J. C. et al. Population density determines the direction of the association between ambient ultraviolet radiation and type 1 diabetes incidence. Pediatric Diabetes, v. 11, n. 6, p. 394–402, set. 2010.
ETTEN, E. VAN; MATHIEU, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. The Journal of Steroid Biochemistry and Molecular Biology, v. 97, n. 1–2, p. 93–101, out. 2005.
FU, Z.; R GILBERT, E.; LIU, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Current diabetes reviews, v. 9, n. 1, p. 25–53, 2013.
GIRI, D. et al. Treating vitamin D deficiency in children with type I diabetes could improve their glycaemic control. BMC Research Notes, v. 10, n. 1, p. 465, 7 dez. 2017.
GRAMMATIKI, M.; KARRAS, S.; KOTSA, K. The role of vitamin D in the pathogenesis and treatment of diabetes mellitus: a narrative review. Hormones, v. 18, n. 1, p. 37–48, 25 mar. 2019.
GRECO, E. A.; LENZI, A.; MIGLIACCIO, S. Role of Hypovitaminosis D in the Pathogenesis of Obesity-Induced Insulin Resistance. Nutrients, v. 11, n. 7, p. 1506, 1 jul. 2019.
GREER, R. M. et al. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatric Diabetes, v. 14, n. 1, p. 31–41, fev. 2013.
HE, L.-P. et al. Progress in the Relationship between Vitamin D Deficiency and the Incidence of Type 1 Diabetes Mellitus in Children. Journal of Diabetes Research, v. 2022, p. 1–8, 2 set. 2022a.
HE, L.-P. et al. Progress in the Relationship between Vitamin D Deficiency and the Incidence of Type 1 Diabetes Mellitus in Children. Journal of Diabetes Research, v. 2022, p. 1–8, 2 set. 2022b.
HIGGINS, J. P. T. Cochrane handbook for systematic reviews of interventions version 5.0. 1. The Cochrane Collaboration. http://www. cochrane-handbook. org, 2008.
HOLICK, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology & metabolism, v. 96, n. 7, p. 1911–1930, 2011a.
HOLICK, M. F. et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, v. 96, n. 7, p. 1911–1930, jul. 2011b.
HYPPÖNEN, E. et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. The Lancet, v. 358, n. 9292, p. 1500–1503, nov. 2001.
INFANTE, M. et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients, v. 11, n. 9, p. 2185, 11 set. 2019.
KRUL-POEL, Y. H. M. et al. MANAGEMENT OF ENDOCRINE DISEASE: The effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. European Journal of Endocrinology, v. 176, n. 1, p. R1–R14, jan. 2017.
LEE, C. J. et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: A systematic review and meta-analysis of intervention studies. Journal of Diabetes and its Complications, v. 31, n. 7, p. 1115–1126, jul. 2017.
LEMOS, M. C. et al. Lack of association of vitamin D receptor gene polymorphisms with susceptibility to type 1 diabetes mellitus in the Portuguese population. Human Immunology, v. 69, n. 2, p. 134–138, fev. 2008.
LIPS, P. et al. Vitamin D and type 2 diabetes. The Journal of Steroid Biochemistry and Molecular Biology, v. 173, p. 280–285, out. 2017.
LITTORIN, B. et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia, v. 49, n. 12, p. 2847–2852, 9 nov. 2006.
LIU, C. et al. Serum vitamin D deficiency in children and adolescents is associated with type 1 diabetes mellitus. Endocrine Connections, v. 7, n. 12, p. 1275–1279, dez. 2018.
LI, X. et al. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients, v. 10, n. 3, p. 375, 19 mar. 2018.
LOVIC, D. et al. The Growing Epidemic of Diabetes Mellitus. Current Vascular Pharmacology, v. 18, n. 2, p. 104–109, 27 jan. 2020.
MAEDA, S. S. et al. Recomendações da Sociedade Brasileira de Endocrinologia e Metabologia (SBEM) para o diagnóstico e tratamento da hipovitaminose D. Arquivos Brasileiros de Endocrinologia & Metabologia, v. 58, p. 411–433, 2014.
MAGER, D. R. et al. Vitamin D3 supplementation, bone health and quality of life in adults with diabetes and chronic kidney disease: Results of an open label randomized clinical trial. Clinical Nutrition, v. 36, n. 3, p. 686–696, jun. 2017.
MAGLIANO, D. J.; BOYKO, E. J. IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th ed. Brussels: International Diabetes Federation, 2021.
MANOUSAKI, D. et al. Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLOS Medicine, v. 18, n. 2, p. e1003536, 25 fev. 2021.
MARETZKE, F. et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases—An Umbrella Review. Nutrients, v. 12, n. 4, p. 969, 31 mar. 2020.
MILLER, K. M. et al. Are low sun exposure and/or vitamin D risk factors for type 1 diabetes? Photochemical & Photobiological Sciences, v. 16, n. 3, p. 381–398, 27 mar. 2017.
MIRHOSSEINI, N. et al. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism, v. 102, n. 9, p. 3097–3110, 1 set. 2017.
MITCHELL, D. M. et al. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high-dose ergocalciferol administration: a randomized controlled trial. The American Journal of Clinical Nutrition, v. 102, n. 2, p. 385–392, ago. 2015.
MOHAMMADNEJAD, Z. et al. Association between vitamin D receptor gene polymorphisms and type 1 diabetes mellitus in Iranian population. Molecular Biology Reports, v. 39, n. 2, p. 831–837, 17 fev. 2012.
MUSAZADEH, V. et al. Effect of vitamin D supplementation on type 2 diabetes biomarkers: an umbrella of interventional meta-analyses. Diabetology & Metabolic Syndrome, v. 15, n. 1, p. 76, 19 abr. 2023.
NAJJAR, L. et al. Vitamin D and Type 1 Diabetes Risk: A Systematic Review and Meta-Analysis of Genetic Evidence. Nutrients, v. 13, n. 12, p. 4260, 26 nov. 2021.
NAPOLI, N. et al. Effect of Calcitriol on Bone Turnover and Osteocalcin in Recent-Onset Type 1 Diabetes. PLoS ONE, v. 8, n. 2, p. e56488, 20 fev. 2013.
NEJENTSEV, S. et al. Analysis of the Vitamin D Receptor Gene Sequence Variants in Type 1 Diabetes. Diabetes, v. 53, n. 10, p. 2709–2712, 1 out. 2004.
NIGIL HAROON, N. et al. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: a systematic review of interventional studies. Journal of Diabetes & Metabolic Disorders, v. 14, n. 1, p. 3, 12 fev. 2015.
OGROTIS, I.; KOUFAKIS, T.; KOTSA, K. Changes in the Global Epidemiology of Type 1 Diabetes in an Evolving Landscape of Environmental Factors: Causes, Challenges, and Opportunities. Medicina, v. 59, n. 4, p. 668, 28 mar. 2023.
OGUNKOLADE, B.-W. et al. Vitamin D Receptor (VDR) mRNA and VDR Protein Levels in Relation to Vitamin D Status, Insulin Secretory Capacity, and VDR Genotype in Bangladeshi Asians. Diabetes, v. 51, n. 7, p. 2294–2300, 1 jul. 2002.
PANI, M. A. et al. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes, v. 49, n. 3, p. 504–507, 1 mar. 2000.
PANJIYAR, R. P. et al. Sustained serum 25-hydroxyvitamin D concentrations for one year with cholecalciferol supplementation improves glycaemic control and slows the decline of residual β cell function in children with type 1 diabetes. Pediatric Endocrinology Diabetes and Metabolism, v. 24, n. 3, p. 111–117, 2018.
PFEIFER, M. et al. Effects of a Short-Term Vitamin D3 and Calcium Supplementation on Blood Pressure and Parathyroid Hormone Levels in Elderly Women1. The Journal of Clinical Endocrinology & Metabolism, v. 86, n. 4, p. 1633–1637, 1 abr. 2001.
PITOCCO, D. et al. The effects of calcitriol and nicotinamide on residual pancreatic β‐cell function in patients with recent‐onset Type 1 diabetes (IMDIAB XI). Diabetic Medicine, v. 23, n. 8, p. 920–923, 26 ago. 2006.
POVALIAEVA, A. et al. Evaluation of Vitamin D Metabolism in Patients with Type 1 Diabetes Mellitus in the Setting of Cholecalciferol Treatment. Nutrients, v. 12, n. 12, p. 3873, 18 dez. 2020.
QI, J.-W. et al. Association Between Plasma Vitamin D2 and Type 2 Diabetes Mellitus. Frontiers in Endocrinology, v. 13, 1 jun. 2022.
QUEIROZ, N. N. M. DE. Efeito da suplementação de altas doses de colecalciferol sobre o comportamento da pressão arterial em pacientes normotensos com diabetes mellitus tipo 1. 2018.
RAMAGOPALAN, S. V. et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Research, v. 20, n. 10, p. 1352–1360, out. 2010.
RASOUL, M. A. et al. Relationship of four vitamin D receptor gene polymorphisms with type 1 diabetes mellitus susceptibility in Kuwaiti children. BMC Pediatrics, v. 19, n. 1, p. 71, 7 dez. 2019.
ROSSINI, M. et al. Dose-Dependent Short-Term Effects of Single High Doses of Oral Vitamin D3 on Bone Turnover Markers. Calcified Tissue International, v. 91, n. 6, p. 365–369, 28 dez. 2012.
SACERDOTE, A. et al. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Current Diabetes Reports, v. 19, n. 10, p. 101, 10 out. 2019.
SAVASTIO, S. et al. Vitamin D Deficiency and Glycemic Status in Children and Adolescents with Type 1 Diabetes Mellitus. PLOS ONE, v. 11, n. 9, p. e0162554, 8 set. 2016.
SCRAGG, R.; SOWERS, M.; BELL, C. Serum 25-Hydroxyvitamin D, Diabetes, and Ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care, v. 27, n. 12, p. 2813–2818, 1 dez. 2004.
SEGOVIA-ORTÍ, R. et al. Vitamin D status is related to severity at onset of diabetes and worse glycemic control. Journal of Pediatric Endocrinology and Metabolism, v. 33, n. 10, p. 1265–1271, 2020.
SENTINELLI, F. et al. The vitamin D receptor (VDR) gene rs11568820 variant is associated with type 2 diabetes and impaired insulin secretion in Italian adult subjects, and associates with increased cardio-metabolic risk in children. Nutrition, Metabolism and Cardiovascular Diseases, v. 26, n. 5, p. 407–413, maio 2016.
SOOY, K. et al. Calbindin-D28k Controls [Ca2+] and Insulin Release. Journal of Biological Chemistry, v. 274, n. 48, p. 34343–34349, nov. 1999.
SZYMCZAK-PAJOR, I.; DRZEWOSKI, J.; ŚLIWIŃSKA, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. International Journal of Molecular Sciences, v. 21, n. 18, p. 6644, 11 set. 2020.
TAKIISHI, T. et al. Vitamin D and Diabetes. Endocrinology and Metabolism Clinics of North America, v. 39, n. 2, p. 419–446, jun. 2010.
THRAILKILL, K. M.; FOWLKES, J. L. The Role of Vitamin D in the Metabolic Homeostasis of Diabetic Bone. Clinical Reviews in Bone and Mineral Metabolism, v. 11, n. 1, p. 28–37, 14 mar. 2013.
TRIPKOVIC, L. et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. The American Journal of Clinical Nutrition, v. 95, n. 6, p. 1357–1364, jun. 2012.
WANG, J. et al. Pediatric diabetes in China: Challenges and actions. Pediatric Diabetes, v. 23, n. 5, p. 545–550, 24 ago. 2022.
WIMALAWANSA, S. J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. The Journal of Steroid Biochemistry and Molecular Biology, v. 175, p. 177–189, jan. 2018.
WOLDEN-KIRK, H. et al. Discovery of Molecular Pathways Mediating 1,25-Dihydroxyvitamin D3 Protection Against Cytokine-Induced Inflammation and Damage of Human and Male Mouse Islets of Langerhans. Endocrinology, v. 155, n. 3, p. 736–747, 1 mar. 2014.
WU, C. et al. Vitamin D supplementation and glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Metabolism, v. 73, p. 67–76, ago. 2017.
ZHANG, Y. et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ, p. l4673, 12 ago. 2019.
TABLES
Table 1 – Description of the PICOS strategy that was used.
PICOS Strategy Description Abbreviation Components Population P T1DM Intervention I VD supplementation Comparison C T1DM subjects receiving intra-individuals VD supplementation or placebo Outcomes O Glycemic profile Study design S Randomized clinical trial
T1DM, type 1 diabetes mellitus; VD, vitamin D
Table 2 – Main characteristics of the studies included in the review.
Conclusions Vitamin D repletion might improve glycemic control in T1DM. Supplementation with alfacalcidol can safely preserve islet beta cell function in newly diagnosed T1DM. Improved metabolic control may be explained by better glucose utilization in muscles. High doses of cholecalciferol supplementation can improve HRQol in patients with T1DM. Glycemic control may not be responsible for the difference found in the evolution of residual B-cell function. Hypo-vitaminosis D significantly improves biological markers (FPG, HbA1c, serum vitD3 and serum insulin). Ergocalciferol reduced serum TNF-α and the rates of increase in HbA1c and IDAA1c, suggesting a protection of GFR and PR in newly diagnosed TDM1. Oral vit D supplementation for 3 months improved levels of serum vit D levels and HbA1C, 87.5% and 86.5% respectively. VD may serve as an adjunct to insulin therapy for T1DM by increasing residual beta cell function and improving insulin secretion. However, it did not reduce HbA1c and the need for exogenous insulin. Treatment with 1,25(OH)2D3 at daily dosage of 0.25 mcg was safe but did note reduce loss of beta-cell function. Main Results Age and 25(OH)D levels at 12 weeks were significantly correlated with better HbA1c levels. There was a non-significant trend for HbA1c to be lower in the I group at months 3 and 6. It was observed a significant decrease in HbA1c levels after VD treatment. A reduction in albuminuria was detected at the end of the trial, without any changes in glycemic control. BMI, HbA1c and insulin requirements were similar between the intervention and control group. All DM patients treated with vit D3 revealed a significant decrease in HbA1c levels after 3 months of treatment. There were no significant differences between the groups during the trial for SCP concentration, HbA1c or total daily dose of insulin. There was a significant improvement in levels of serum vit D and decrease of HbA1C after the administration of vit D. Additionally, vit D and HbA1c showed a significant negative correlation There was no significant difference in HbA1c, and insulin requirement at six months between the two groups. HbA1C and daily insulin requirements were similar between treatment and placebo groups throughout the study follow-up period. Study Characteristics 51 ♀ and 29 ♂ (21.0 ± 7.6) Patients having 25-OH D < 50nmol/L received 4000 IU of VD I: alfacalcidol 0,50mg/day (22 ♀ and 7 ♂) C: placebo capsule (17 ♀ and 8 ♂) 19 ♀ and 20 ♂ Patients received 4000 IU/day of VD under a double blind crossover of 2 phases of treatment (G1: 21 and G2: 18) 33 ♀ and 31 ♂ Patients having 25-OH D < 30ng/mL received 10.000 IU of VD and those with levels between 30-60 ng/mL 4.000 IU/day. I: Cholecalciferol 2000 IU/day (19 participants C: placebo capsule (19 participants) G1: 25 patients received vit. D3 2000 IU/day G2: 25 patients received insulin twice daily (soluble and lente) G3: 25 patients were considered control group Ergocalciferol 50000 IU / week for 2 months then 25000 IU / week for 10 months. I: 8 ♀ and 10 ♂
C: 4 ♀ and 14 ♂Cholecalciferol 2800 IU / day for 3 months. 25♀ and 20 ♂ I: Cholecalciferol 60000 IU / month (14 ♀ and 12 ♂) C: standard care (13 ♀ and 13 ♂) I: 20 C: 18 0.25 mcg VD or placebo daily for 9 months and followed for a total of 18 months for safety. Reference Aljabri et al. (2010) Ataie-Jafari et al. (2013) Bogdanou et al. (2017) de Souza et al. (2022) Gabbay et al. (2012) Nafei et al. (2017) Nwosu et al. (2022) Ragab et al. (2021) Sharma et al. (2017) Walter et al. (2010)
VD: vitamin D; HbA1c: Hemoglobin glycated; T1DM: Type 1 diabetes mellitus; BMI: body mass index; FPG: Fasting plasma glucose; TNF-α: tumor necrosis factor alpha; IDAA1c: insulin dose–adjusted A1c; RBCF: residual β-cell function; PR: partial clinical remission; SCP: stimulated C-peptide; 1,25(OH)2D3: 1,25-dihydroxyvitamin D3.
Table 3 – Information on authors, countries and sample characteristics of included studies.
Reference Country Number Age (Years) BMI (Kg/m²) Gender (F/M) Mean ± SD Aljabri et al. (2010) Saudi Arabia 80 21 ± 7.6 22.3 ± 4.0 51 / 29 Ataie-Jafari et al. (2013) Iran 54 I: 10.2 ± 2.5 C: 11.1 ± 1.6 NR 39 / 15 Bogdanou et al. (2017) German 39 44 SD 24.6 SD 19 / 20 de Souza et al. (2022) Brazil 64 27.6 ± 10.1 24.0 ± 4 33 / 31 Gabbay et al. (2012) Brazil 35 I: 13.5 ± 5.1 C: 12.5 ± 4.8 NR 15 / 20 Nafei et al. (2017) Iraq 75 G1: 8.78 ± 0.53 G2: 8.16 ± 0.54 G3: 6.77 ± 0.49 NR NR Nwosu et al. (2022) USA 36 I: 13.25 ± 2.76 C: 14.28 ± 2.86 I: 22.03 ± 5.41 C: 22.01 ± 4.15 12 / 24 Ragab et al. (2021) Epygt 45 NR NR 25 / 20 Sharma et al. (2017) India 52 I: 9.5 ± 3.9 C: 9.0 ± 4.4 I: 22.6 SD
C: 24.2 SD25 / 27 Walter et al. (2010) Germany 38 18 – 39 r NR NR
BMI: Body mass index; F: Female; M: Male; I: Intervention; C: Control; r: range; SD: Standard deviation not reported; NR: Not reported.
Table 4 – Effect of VD supplementation in T1DM patients before and after supplementation.
Reference Patients (A / T) Effect on HbA1C Aljabri et al. (2010) 54 / 80 Unchanged Ataie-Jafari et al. (2013) 29 / 54 Improvement Bogdanou et al. (2017) 39 / 39 Improvement de Souza et al. (2022) 64 / 64 Unchanged Gabbay et al. (2012) 17 / 35 Unchanged Nafei et al. (2017) 25 / 75 Improvement Nwosu et al. (2022) 18 / 36 Unchanged Ragab et al. (2021) 45 / 45 Improvement Sharma et al. (2017) 26 / 52 Unchanged Walter et al. (2010) 20 / 38 Improvement
A = Affected; T = Total; HbA1c = Hemoglobin Glycated
Table 5 – Information on the interventions and variables analyzed.
Reference Baseline VD VD type Dose & Frequency Study Duration Improvement (n=5) Ataie-Jafari et al. (2013) I: 13.9 ± 6.0 ng/mL
C: 12.5 ± 6.4 ng/mLAlfacalcidol 20 IU / day 6 months Bogdanou et al. (2017) 18 ng/mL m Cholecalciferol 4000 IU / day 6 months Nafei et al. (2017) G1: 11.05 ± .09 ng/mL
G2:15.0 ± 1.56 ng/mL G3(C):26.67 ± 2.20 ng/mLCholecalciferol G1: 2000 IU / day 3 months Ragab et al. (2021) 11.4 ± 3.4 ng/mL Cholecalciferol 2800 IU / day 3 months Walter et al. (2010) 24.6 SD pg/mL Calcitriol 0.25 µg / day 18 months Unchanged (n=5) Aljabri et al. (2010) <35.4 nmol/L: 32.5% 35.4-51 nmol/L: 33.8% >51 nmol/L: 33.8% Cholecalciferol 4000 IU / day 3 months de Souza et al. (2022) 26.7 ± 9.0 ng/mL Cholecalciferol VD <30: 10000 IU / day VD 30-60: 4000 / day 3 months Gabbay et al. (2012) I: 26.34 ± 6.49 ng/mL C: 25.76 ± 5.68 ng/mL Cholecalciferol 2000 IU / day 18 months Nwosu et al. (2022) 22 SD ng/mL Ergocalciferol 50000 IU / week (2 months) 25000 IU / week (10 months) 12 months Sharma et al. (2017) I: 20.7 ± 10.5 ng/mL
C:19.7 ± 11.8 ng/mLCholecalciferol 60000 IU / month 6 months
I: Intervention; C: Control; G: group; SD: Standard deviation not reported; m: median; IU: international units.
FIGURES
Figure 1 – Flow diagram of Search and selection of studies.
Figure 2 – Risk of bias summary: review author’s judgments about each risk of bias item for each included study.
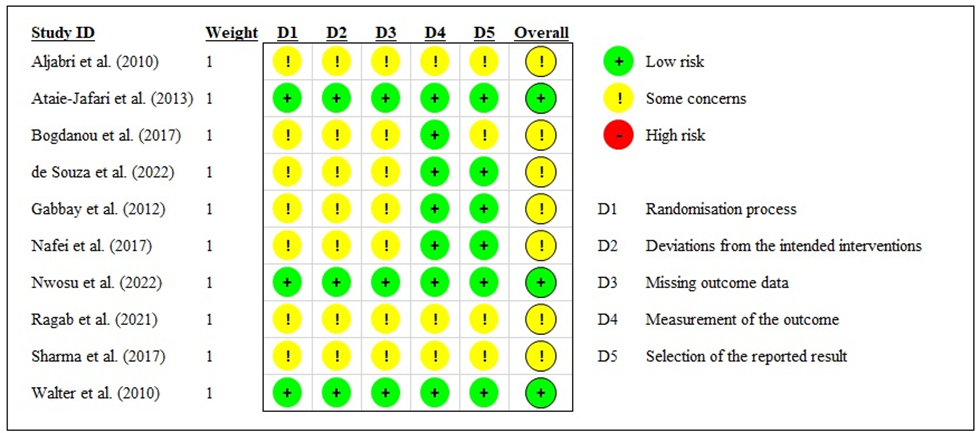
Figure 3 – Geographical distribution of the participating countries in the analyzed studies.
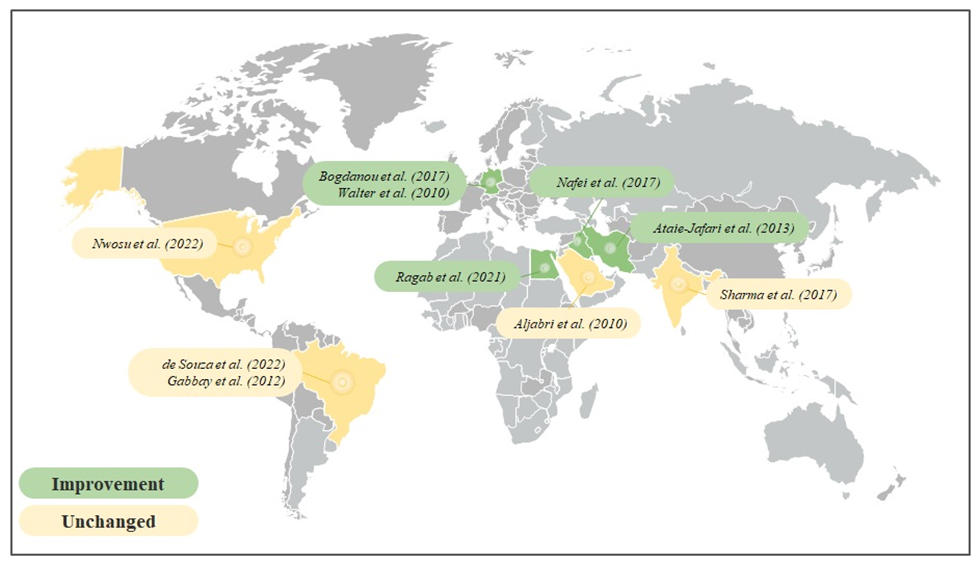
Figure 4 – Baseline and follow-up levels of vitamin D from each study.
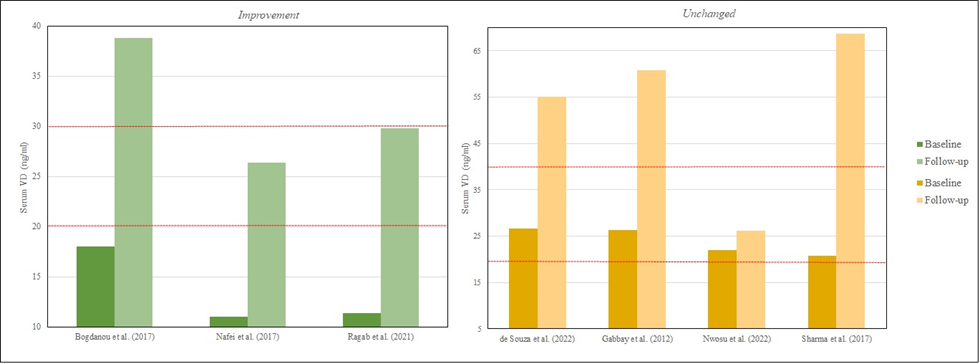
Figure 5 – Effect of vitamin D supplementation on Hemoglobin A1c according to baseline vitamin D score