CAR-T: O FUTURO DA IMUNOTERAPIA
REGISTRO DOI: 10.69849/revistaft/ar10202502021327
Rosa, Marcely Diner dos Reis¹;
Gatti, Luciano Lobo²;
Silva, Douglas Fernandes³.
Abstract
Introduction: Immunotherapy has transformed cancer treatment by leveraging historical observations of spontaneous tumor regression linked to infections. Pioneers like Fehleisen and Coley laid the groundwork for utilizing the immune system against cancer, contrasting with traditional treatments by targeting tumors with reduced side effects. Objective: This study aims to explore the efficacy of CAR-T cell therapy in cancer treatment and evaluate the role of biomedical professionals in its implementation and monitoring. CAR-T therapy involves genetically modifying a patient’s T cells to enhance their ability to recognize and eliminate cancer cells, offering a promising avenue for advancing cancer treatment. Methods: The methodology involved a comprehensive review of current literature on CAR-T therapy, focusing on its structural components, mechanisms of action, clinical applications across various cancers (such as ALL, DLBCL, and MM), and associated challenges in safety and toxicity management. Results: CAR-T therapy has demonstrated significant efficacy in treating refractory leukemia and lymphomas, achieving complete remission in some cases. The therapy involves genetically modifying T cells to express chimeric antigen receptors (CARs) that target specific antigens on cancer cells. This activation triggers immune responses leading to tumor cell destruction, supported by the release of cytokines and induction of apoptosis. Despite notable successes, challenges such as cytokine release syndrome and the need for improved targeting strategies remain significant. Conclusion: CAR-T therapy represents a pivotal advancement in cancer treatment, offering personalized and effective options for patients previously resistant to conventional therapies. The ongoing evolution of CAR-T technology and collaborative efforts among researchers, healthcare providers, and regulatory bodies are crucial for enhancing its safety, efficacy, and accessibility worldwide.
Keywords: CAR-T, Therapy, Immunotherapy.
Resumo
Introdução: A imunoterapia transformou o tratamento do câncer ao aproveitar observações históricas de regressão espontânea de tumores associada a infecções. Pioneiros como Fehleisen e Coley lançaram as bases para utilizar o sistema imunológico contra o câncer, contrastando com tratamentos tradicionais ao direcionar tumores com efeitos colaterais reduzidos. Objetivo: Este estudo visa explorar a eficácia da terapia com células CAR-T no tratamento do câncer e avaliar o papel dos profissionais biomédicos na sua implementação e monitoramento. A terapia com células CAR-T envolve modificar geneticamente as células T do paciente para aumentar sua capacidade de reconhecer e eliminar células cancerígenas, oferecendo uma promissora via para avançar no tratamento do câncer. Métodos: A metodologia envolveu uma revisão abrangente da literatura atual sobre terapia com células CAR-T, focando em seus componentes estruturais, mecanismos de ação, aplicações clínicas em vários tipos de câncer (como LLA, DLBCL e MM) e os desafios associados na gestão da segurança e toxicidade. Resultados: A terapia com células CAR-T demonstrou eficácia significativa no tratamento de leucemias e linfomas refratários, alcançando remissão completa em alguns casos. A terapia envolve a modificação genética das células T para expressar receptores de antígenos quiméricos (CARs) que direcionam antígenos específicos em células cancerígenas. Essa ativação desencadeia respostas imunes que levam à destruição das células tumorais, suportada pela liberação de citocinas e indução de apoptose. Apesar dos sucessos notáveis, desafios como a síndrome de liberação de citocinas e a necessidade de estratégias de direcionamento melhoradas permanecem significativos. Conclusão: A terapia com células CAR-T representa um avanço fundamental no tratamento do câncer, oferecendo opções personalizadas e eficazes para pacientes previamente resistentes às terapias convencionais. A evolução contínua da tecnologia CAR-T e esforços colaborativos entre pesquisadores, profissionais de saúde e órgãos reguladores são cruciais para aprimorar sua segurança, eficácia e acessibilidade em todo o mundo.
Palavras-chave: CAR-T, Terapia, Imunoterapia.
1. INTRODUCTION
Immunotherapy is a very recent medical achievement, originating a few decades ago. However, from ancient Egypt around 3000 years ago to the early 19th century, there were several anecdotal reports of tumors disappearing spontaneously or after an infection with concomitant high fever. The first scientific forays into modulating patients’ immune systems to combat cancer can be attributed to two German doctors, Fehleisen and Busch, who independently observed a remarkable tumor regression after an erysipelas infection (DOBOSZ; DZIECIĄTKOWSKI, 2019).
Continuing their studies, Dr. Fehleisen correctly identified the bacterial strain responsible for erysipelas and tumor reduction as Streptococcus pyogenes. Subsequent advances came from William Bradley Coley, known today as the “Father of Immunotherapy,” who made his first attempt to use the immune system to treat bone cancer in 1891 (DOBOSZ; DZIECIĄTKOWSKI, 2019).
The immune system plays a vital role in protection against invaders such as viruses and bacteria. This complex system consists of a variety of specialized cells, each with specific functions (OTONI, 2022). The cells that make up the immune system, leukocytes, include lymphocytes, neutrophils, and macrophages, among others. These cells play crucial roles in identifying and eliminating invading agents. For example, lymphocytes are responsible for producing antibodies, which are proteins capable of recognizing and neutralizing pathogens. When an infection occurs, lymphocytes produce specific antibodies to combat the invader. In addition to lymphocytes, macrophages and neutrophils are also important in the body’s defense, acting in the cellular immune response. These cells can directly attack pathogen-infected cells (OTONI, 2022).
Cancer, a condition characterized by malignant tumors due to abnormal cellular differentiation and rapid multiplication, is one of the leading causes of death worldwide, resulting in approximately 10 million deaths in 2020 (ATTY JEANE TOMAZELLI, 2020). In the therapeutic scenario of 1949, immunotherapy emerged as an effective and emerging approach, encompassing different modalities such as immune checkpoint inhibitors, monoclonal antibodies, cancer vaccines, adoptive cell transfer, and the use of cytokines (FALÇONI JÚNIOR, 2020).
Immunotherapy stimulates a specific immune response against tumor cells, aiming to inhibit and destroy cancer in a targeted manner while protecting the organism from harm. Unlike traditional treatments, immunotherapy does not directly target cancer cells but mobilizes the immune system to recognize and combat tumors, resulting in fewer side effects and a satisfactory safety profile. With advancements in the understanding of tumor immunology and continuous technological development, immunotherapy is expected to become a fundamental part of cancer treatment, offering new perspectives for effectively managing this devastating disease (DOBOSZ; DZIECIĄTKOWSKI, 2019).
CAR-T cell therapy (Chimeric Antigen Receptor T cells) is an advanced immunotherapy approach used to treat cancer (ABBAS; LICHTMAN; PILLAI, 2019). This technique involves several complex steps, from collecting the patient’s T lymphocytes, genetic modification in the laboratory, and infusion of these cells back into the patient. The history of CAR-T therapy spans over 60 years of evolution and pioneering efforts towards cancer cure. In 1950, the first bone marrow transplant, which is also a type of cellular therapy, took place. Since then, numerous discoveries have been made in the world of science and health (ROCHA, 2018).
In 2010, two individuals diagnosed with advanced-stage chronic lymphoblastic leukemia voluntarily participated in the pioneering clinical trial of CAR-T therapy conducted at the University of Pennsylvania School of Medicine (KALOS et al., 2011).
CAR-T cell therapy (Chimeric Antigen Receptor T cells) is an advanced immunotherapy approach used to treat cancer. According to the same authors, this technique involves several complex steps, from collecting the patient’s T lymphocytes, genetic modification in the laboratory, and infusion of these cells back into the patient.
The therapy using Chimeric Antigen Receptor T cells (CAR-T) has significantly transformed the therapeutic approach of immunotherapy in combating cancer. It has proven to be highly effective in treating different types of blood cancers and the lymphatic system. It is considered a modified drug, in which T lymphocytes that are part of the immune system undergo genetic alterations, having their functions enhanced to help them detect and eliminate tumor cells independently of the Major Histocompatibility Complex (MHC) (HUANG et al., 2023).
The objective of this study was to investigate through updated literature the efficacy of CAR-T cells (Chimeric Antigen Receptor T cells) in treating cancer patients and to evaluate the role of biomedical professionals in implementing and monitoring this innovative therapy, highlighting its importance in advancing medicine and improving public health.
2. METHODOLOGY
This study was conducted through a literature review, where studies were meticulously chosen after comprehensive research conducted in various electronic databases, including PubMed (National Library of Medicine), Lilacs (Latin American and Caribbean Literature in Health Sciences), Scielo (Scientific Electronic Library Online), and Google Scholar.
The database search was conducted between February and May 2024 with the central theme: “CAR-T Cells, The Future of Immunotherapy.” The keywords used for the search included: “advanced genetic technologies,” “history of CAR-T cell therapy,” “advanced immunotherapy,” “CAR-T cell engineering,” and “Immunotherapy.” These keywords were combined with relevant terms, such as clinical application, molecular diagnosis, treatment of genetic and genomic diseases, to provide a comprehensive view of innovations in genetic technologies in the health field and their relation to the biomedical field.
For the research development, articles selected from journals according to the theme mentioned above, published in English and Portuguese, were used. Articles not relevant to the proposed theme were rejected.
3. CAR-T THERAPY (CHIMERIC ANTIGEN RECEPTOR T-CELL)
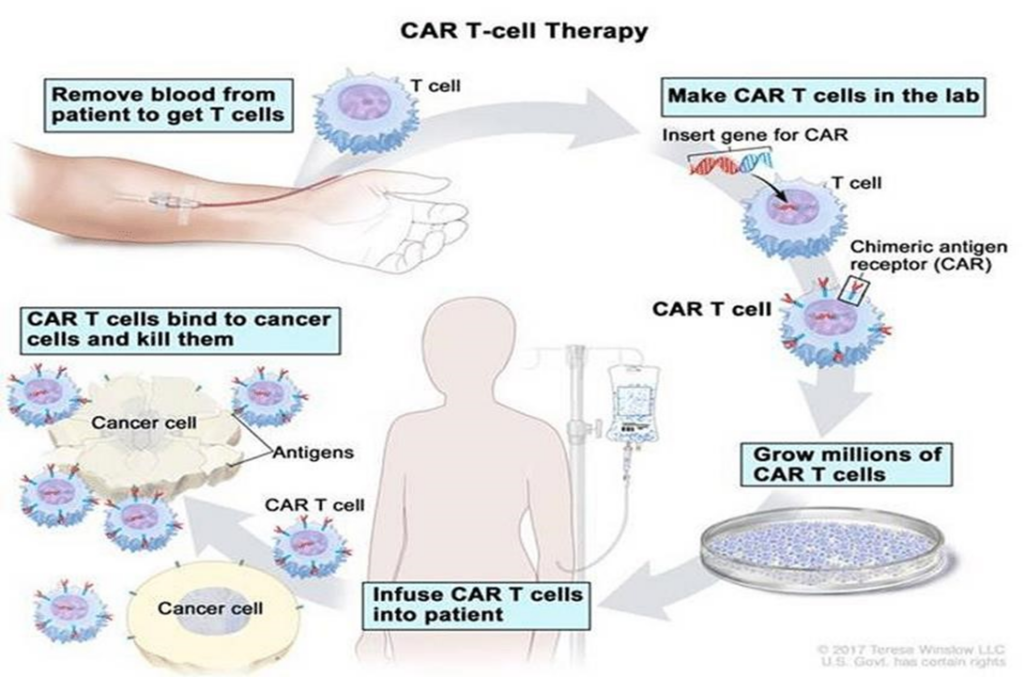
CAR-T therapy (Chimeric Antigen Receptor T-cell) represents a revolutionary advancement in cancer treatment. Figure 1 shows that this technique allows the patient’s own immune cells to be reprogrammed to recognize and precisely target cancer cells (KUFEL; LEWANDOWSKI, 2023).
The importance of CAR-T therapy, according to Zhang et al., Biomarker Research (2017), lies in its ability to offer a highly specific and effective approach against various types of cancer, especially those that do not respond to conventional treatments like chemotherapy and radiotherapy. Additionally, CAR-T therapy offers the possibility of longer-lasting treatments with fewer side effects compared to traditional options (ZHANG et al., 2017).
This technique has shown promising results in patients with refractory leukemia and lymphomas, providing significant response rates and even leading to complete remission of the disease in some cases. Below is a detailed review of this advanced and innovative technology (KUFEL; LEWANDOWSKI, 2023).
3.1. STRUCTURE AND MECHANISMS OF ACTION:
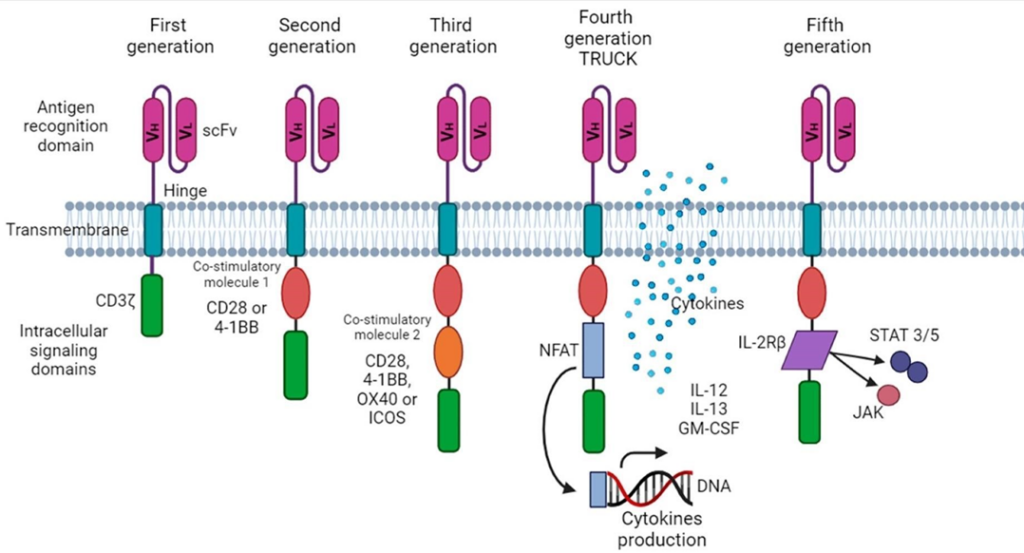
CAR-T cells, derived from the patient’s own CD8+ T lymphocytes and genetically modified to recognize tumor antigens, have been developed in four generations. As shown in Figure 2, in the first generation, T cells are extracted from the patient, modified in the laboratory, and reinfused into the patient after expansion. In the second, donor T cells are modified and infused into the patient. The third generation includes costimulatory molecules to enhance the antitumor response, while the fourth, called TRUNKs, release cytokines to amplify the immune response in solid tumors (LIM; JUNE, 2017).
When the chimeric antigen receptor (CAR) connects to the antigen present on the cancer cell, the modified T cell is activated, triggering a cascade of signals within the cell. This activation process leads to the release of cytokines, such as interferon-gamma (a glycosylated protein and a potent multifunctional cytokine) and interleukin-2 (an interleukin that induces the maturation of B and T lymphocytes), which recruit other immune system cells and promote an antitumor response. Additionally, CAR-T cells induce cancer cell death through different mechanisms, such as releasing cytotoxic substances or inducing apoptosis in tumor cells. After recognizing and attacking cancer cells, some CAR-T cells can multiply and differentiate into memory cells, providing a long-lasting immune response and protection against cancer recurrence. This development aims to offer a highly effective therapy for patients facing cancers resistant to conventional treatments (PEREIRA, 2019).
According to Otoni (OTONI, 2022), in the context of CAR-T cell therapy, the genetic engineering process typically involves using viral vectors, such as retroviruses or lentiviruses, to insert a transgene encoding the chimeric antigen receptor into the patient’s T cells. The selected antigen for CAR-T cell therapy is commonly CD19, present on the surface of B lymphocytes. For example, in acute lymphoblastic leukemia, B lymphocytes express this antigen on their surface. The CAR protein can recognize CD19, binding to it, and triggering a robust immune response against cancer cells.
3.2. CLINICAL APPLICATIONS OF CAR-T THERAPY (TYPES OF CANCER AND TARGET ANTIGENS)
According to Roberto C Sterner (ROBERTO C STERNER, 2021), the types of cancer treatable with CAR-T therapy are: Acute Lymphoblastic Leukemia, Diffuse Large B-Cell Lymphoma (DLBCL) and Multiple Myeloma (MM). CAR-T therapy, according to the same authors, was initially developed to treat ALL, a white blood cell cancer that mainly affects children and young adults.
CD19 antigen is a common target for CAR-T therapies in ALL, Diffuse Large B-Cell Lymphoma (DLBCL) is an aggressive form of non-Hodgkin’s lymphoma, where CAR-T therapy has demonstrated remarkable efficacy, especially in patients who do not respond to conventional treatments. Multiple Myeloma (MM) is also another example, which in turn is a plasma cell cancer that occurs in the bone marrow and has been an emerging target for CAR-T therapy (ROBERTO C STERNER, 2021).
Antigens such as BCMA (B myeloma cell antigen) are investigated as targets for this treatment modality. As is Acute Myeloid Leukemia (AML), which although less common than ALL, is another form of leukemia that is being explored as a target for CAR-T therapy in clinical studies (ROBERTO C STERNER, 2021).
Hongwen Li et al. (LI et al., 2023) developed a second-generation CAR construct and produced CAR-T cells targeting the CD38 molecule. Subsequently, they evaluated the effects of CAR-T cells on MM cell lines. It was then observed that CD38-CAR-T cells showed greater cytotoxicity against MM cell lines and primary MM cells than control T cells in in vitro tests. Even at an effector-to-target ratio of 1:100, more than 50% of MM1.s and RPMI8226 cells were eliminated by CAR-T cells. Furthermore, CAR-T cells demonstrated increased cytotoxicity against primary MM cells. It was also noted that CAR-T cells could be activated and produced a variety of cytokines in a target-dependent manner. In vivo test results also indicated that CART cells exhibited a significant antitumor effect in xenotransplanted mouse models.
Chiara Magnani et al. (MAGNANI et al., 2023) explored in their studies a non-viral technique to interrupt the activity of CAR T cells, aiming to avoid graft rejection. They then used two different strategies: the temporary introduction of an anti-CD117 CAR by mRNA and the generation of CAR T cells with an inducible Caspase 9 safety switch. Both approaches have shown efficacy in eliminating leukemic and healthy cells in animal models. This method offers a promising prospect for initial studies in patients with acute myeloid leukemia before hematopoietic stem cell transplantation.
Philiph B et al. (PHILIP et al., 2014) created a compact marker/suicide gene for T cells by joining target regions of CD34 and CD20 antigens (called RQR8). This gene enables selection using the CliniMACS CD34 system, which is clinically approved by Miltenyi.
Furthermore, the RQR8 gene binds to rituximab, a common pharmaceutical antibody, resulting in the selective removal of cells expressing the transgene. Tests were also carried out to verify the effectiveness of RQR8 both in laboratory conditions and in animal models, as well as in combination with other T cell engineering techniques. We believe that RQR8 will make T cell gene therapy safer and more accessible.
The search for new molecular targets, such as CD38, CD138, SLAMF7, GPRC5D and B cell maturation antigen, is essential to develop effective therapies against refractory/relapsed multiple myeloma using CAR-T cells. These selective targets in cancer cells, but not normal cells, offer promise for more effective treatment and overcoming the challenges of therapy resistance. The incorporation of these targets expands the potential of CAR-T technology for diverse tumor types. Furthermore, strategies such as multivalent CAR-T cells and combinations with other therapeutic modalities are being explored to improve efficacy and reduce tumor resistance (YANG; WANG; ZHOU, 2019).
3.3. CHALLENGES AND LIMITATIONS (SAFETY AND TOXICITY)
Despite the risks and post-infusion cytotoxic effects associated with CAR-T cell therapy, these do not make the technique unfeasible, as they are controllable and considered acceptable in comparison with the expected benefits. Accurate characterization of the infusion dose is crucial to determine the relationship with adverse effects. Advances in research are essential to improve safety, reduce cytotoxic effects and production costs, making therapy more accessible. The development of new CAR-T vectors may increase the feasibility and prognosis of treatment. Study-associated mortality is low, and deaths are not linked to therapy, highlighting the viability of CAR-T cell immunotherapy as a promising therapeutic intervention (SOARES WILLIAM, 2018).
To ensure efficacy and safety, it is necessary to carefully select the targets of CAR-T cell therapy, allowing a more precise approach to cancer. Although initially more effective in hematological cancers, recent research is focused on developing CAR-T therapies for solid tumors, such as those of the lung, breast and pancreas. These advances aim to overcome unique challenges such as the immune barrier and tumor heterogeneity. One promising strategy involves CAR-T cells that target multiple tumor antigens to overcome heterogeneity and reduce the risk of resistance. Researchers are also exploring new approaches to improve efficacy and safety, including additional co-stimulation, genetic modification to prolong persistence, and the development of safety switches to control CAR-T cell activity (AJEJE; DE ALMEIDA; FERNANDES, 2023).
3.4. FUTURE PERSPECTIVES AND THEIR RELATIONSHIP WITH BIOMEDICAL
To further improve therapy, it is necessary to develop new therapeutic strategies to treat a variety of cancers, both solid and hematological. We also seek to improve the safety and efficacy of treatment by better understanding the mechanisms of toxicity and resistance associated with CAR-T therapy.
Personalizing treatment is another important focus, adapting CAR-T therapy to each patient’s individual characteristics, such as genetic profile and immunological response. Reducing costs and increasing accessibility is also a goal, making treatment more accessible to a wider range of patients and healthcare systems. The development of next-generation CAR-T therapies, including multi-target cells, is an area of focus. Furthermore, it is critical to develop more effective approaches to manage adverse side effects such as cytokine release syndrome. Long-term studies are essential to evaluate the durability of responses to CAR-T treatment and its long-term effects on patients’ health (ENRIQUEZ-RODRIGUEZ et al., 2024).
Each CAR-T treatment can be tailored to suit the individual characteristics of each patient’s tumor, considering the genetic and molecular variety of different cancer types. Furthermore, the ability to tune CAR-T cell properties, such as choosing target antigens, including costimulators, and genetic modification to increase cell persistence, provides an additional opportunity to personalize treatment and maximize its effectiveness. This personalized approach has the potential to improve clinical outcomes and reduce side effects, resulting in a more effective and tolerable therapy for patients (DALTRO AMANDA, 2024).
Biomedical scientists play a crucial role in the CAR-T therapy process, as in research and development (CFBM, 2002) thus contributing to advances in new technologies and methods, exploring molecular targets, genetic engineering techniques and strategies to improve the efficacy and safety of the therapy.
These professionals can also participate in production and manufacturing, as they participate in the laboratory production of CAR-T cells, from modifying the T cells to preparing therapeutic doses. In Assessment and Monitoring, in accordance with the council’s regulations (CFBM, 2021) laboratory tests can be carried out to monitor the patient’s immunological response, identify possible adverse effects and evaluate effectiveness throughout the treatment. And they share knowledge with other health professionals, creating educational materials, conducting workshops and ensuring constant updates on advances in the area.
The biomedical role is fundamental in the research, development and clinical application of new technologies in gene therapy, as this professional is actively involved in research and development of new technologies, in genetic counseling, in the administration of clinics and study and research centers, in addition to being a promoter of science education, through dissemination to the scientific and medical community and the public (CARNEIRO, 2021).
4. CONCLUSIONS
CAR T therapy which consists of modifying T cells with chimeric antigen receptors, has emerged as an innovative approach in the treatment scenario against forms of cancer, such as leukemias and refractory lymphomas. During this research, the scientific principles, technological advances and clinical advances that shaped this therapy were investigated. This analysis also revealed that CAR T therapy offers promising results, providing long-lasting responses and, in many cases, even potential cures for patients who did not respond to conventional treatments. However, there are also significant challenges related to the topic, such as toxicities associated with treatment, high costs and the need to improve the effectiveness and persistence of therapeutic responses. It is evident that it is crucial to continue research and develop new approaches to overcome these challenges and expand the therapeutic potential of CAR T therapy. This includes improvements in CAR T technology, exploration of new antigenic targets, optimization of treatment regimens, and identification of strategies to minimize toxicities. Furthermore, successful implementation of CAR T therapy requires a multidisciplinary approach, involving collaborations between researchers, healthcare professionals, regulatory bodies and the pharmaceutical industry. It is also essential to consider ethical, social and economic issues related to equitable access to these innovative therapies. As advances are made in CAR T therapy, it is essential to continue investing in research and resources to harness its full potential and transform cancer treatment. With continued dedication and global collaboration, we can achieve significant advances in the fight against cancer and offer renewed hope to patients and their families.
5. Acknowledgements
The authors wish to University Center of the Integrated Faculties of Ourinhos – Unifio, Ourinhos, SP, Brazil.
6. References
ABBAS, A. K.; LICHTMAN, A. H.; PILLAI, S. Imunologia Celular e Molecular. . [S.l: s.n.], 2019.
AJEJE, P.; DE ALMEIDA, M. E.; FERNANDES, V. TERAPIA COM CÉLULAS CAR-T EM
CÂNCER HEMATOLÓGICO: PROGRESSOS RECENTES E PERSPECTIVAS FUTURAS. Open Science Research XIII. [S.l.]: Editora Científica Digital, 2023. p. 112–113. Disponível em: <http://www.editoracientifica.com.br/articles/code/231014749>.
ATTY JEANE TOMAZELLI, A. Ministério da Saúde Secretaria de Atenção à Saúde Instituto Nacional de Câncer José Alencar Gomes da Silva RELATÓRIO DO INTERVALO ENTRE
DIAGNÓSTICO E INÍCIO DO TRATAMENTO DO CÂNCER NO SUS Dados do PAINEL- Oncologia. [S.l: s.n.], 2020. Disponível em: <https://rbc.inca.gov.br/revista/index.php/revista/article/view/827>.
CARNEIRO, A. C. A IMPORTÂNCIA DA PESQUISA EM BIOMEDICINA: DESENVOLVIMENTO CIENTÍFICO, TECNOLÓGICO E A INDÚSTRIA BIOMÉDICA. . [S.l: s.n.], 2021.
CFBM. SERVIÇO PÚBLICO FEDERAL CONSELHO FEDERAL DE BIOMEDICINA-CFBM. [S.l: s.n.], 2002.
CFBM. SERVIÇO PÚBLICO FEDERAL CONSELHO FEDERAL DE BIOMEDICINA-CFBM. [S.l: s.n.], 2021.
DALTRO AMANDA. Potencial para personalização do tratamento com CAR-T. 2024.
DOBOSZ, P.; DZIECIĄTKOWSKI, T. The Intriguing History of Cancer Immunotherapy. Frontiers in Immunology. [S.l.]: Frontiers Media S.A. , 17 dez. 2019
ENRIQUEZ-RODRIGUEZ, L. et al. Expanding the horizon of transient CAR T therapeutics using virus-free technology. Biotechnology Advances, v. 72, p. 108350, maio 2024.
FALÇONI JÚNIOR. Imunoterapia – uma revisão sobre os novos horizontes no combate ao câncer. Imprensa médica, v. 24, n. 423, p. 76–78, 2020.
HUANG, S. et al. Deciphering and advancing CAR T-cell therapy with single-cell sequencing technologies. Molecular Cancer. [S.l.]: BioMed Central Ltd. , 1 dez. 2023
KALOS, M. et al. L E U K E M I A T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. . [S.l: s.n.], 2011. Disponível em: <https://www.science.org>.
KUFEL, J.; LEWANDOWSKI, P. Nowoczesne techniki w diagnostyce i leczeniu chorób onkologicznych Book · July 2023 CITATIONS 0 READS 800. . [S.l: s.n.], 2023. Disponível em: <https://www.researchgate.net/publication/372647077>.
LI, H. et al. A second-generation CD38-CAR-T cell for the treatment of multiple myeloma. Cancer Medicine, v. 12, n. 9, p. 10804–10815, 1 maio 2023.
LIM, W. A.; JUNE, C. H. The Principles of Engineering Immune Cells to Treat Cancer. Cell. [S.l.]: Cell Press. , 9 fev. 2017
MAGNANI, C. F. et al. Anti-CD117 CAR T cells incorporating a safety switch eradicate human acute myeloid leukemia and hematopoietic stem cells. Molecular Therapy Oncolytics, v. 30, p. 56–71, 21 set. 2023.
OTONI, T. IMUNOLOGIA BÁSICA: UMA REVISÃO APLICADA A ESTUDANTES CARLA PEREIRA FIUZA RODRIGUES. [S.l: s.n.], 2022.
PEREIRA, V. DA C. Definição das terapias celulares com receptores de antígenos quiméricos (CAR), receptores de células t (TCR) e linfócitos infiltrantes de tumor (TIL). Perspectivas futuras para a cura do câncer. Brazilian Journal of health Review, 2019. Disponível em: <https://ojs.brazilianjournals.com.br/ojs/index.php/BJHR/article/view/1307>. Acesso em: 21 abr. 2024.
PHILIP, B. et al. A highly compact epitope-based marker/suicide gene for easier and safer Tcell therapy. 2014. Disponível em: <http://ashpublications.org/blood/articlepdf/124/8/1277/1382207/1277.pdf>.
ROBERTO C STERNER, R. M. S. Terapia com células CAR-T: limitações atuais e estratégias potenciais. 2021.
ROCHA, M. C. DE S. TERAPIA COM CÉLULAS CAR-T: UM AVANÇO NA IMUNOONCOLOGIA. 2018.
SOARES WILLIAM. CENTRO UNIVERSITÁRIO DE BRASÍLIA FACULDADE CIÊNCIAS DA EDUCAÇÃO E SAÚDE GRADUAÇÃO EM BIOMEDICINA TRATAMENTO CONVENCIONAL E A IMUNOTERAPIA DE CÉLULAS CAR-T NA REMISSÃO DE NEOPLASIAS LINFOIDE E MIELOIDE. . [S.l: s.n.], 2018.
TALLANTYRE, E. C. et al. Neurological updates: neurological complications of CAR-T therapy. Journal of Neurology, v. 268, n. 4, p. 1544–1554, 2 abr. 2021. Disponível em: <http://link.springer.com/10.1007/s00415-020-10237-3>.
USCANGA-PALOMEQUE, A. C. et al. CAR-T Cell Therapy: From the Shop to Cancer Therapy. International Journal of Molecular Sciences, v. 24, n. 21, p. 15688, 28 out. 2023.
YANG, X.; WANG, G. XIANG; ZHOU, J. FENG. CAR T Cell Therapy for Hematological Malignancies. Current Medical Science, v. 39, n. 6, p. 874–882, 1 dez. 2019.
ZHANG, C. et al. Engineering CAR-T cells. Biomarker Research. [S.l.]: BioMed Central Ltd, 2017
¹Marcely Diner dos Reis Rosa (ORCID: 0009-0006-7858-9398). E-mail: marcelyydr@gmail.com (Corresponding author)
Department of Biomedicine – University Center of the Integrated Faculties of Ourinhos – Unifio, Ourinhos, SP, Brazil.;
²Luciano Lobo Gatti (ORCID: 0000-0003-2723-3173). E-mail: lobogatti@unifio.edu.br
Department of Biomedicine – University Center of the Integrated Faculties of Ourinhos – Unifio, Ourinhos, SP, Brazil.;
³Douglas Fernandes da Silva (ORCID: 0000-0002-0252-1112). E-mail: douglas.silva@unifio.edu.br
Department of Biomedicine – University Center of the Integrated Faculties of Ourinhos – Unifio, Ourinhos, SP, Brazil..
