ALÉM DA SUPERFÍCIE: DESAFIOS METODOLÓGICOS NA AVALIAÇÃO DOS RISCOS DO USO DE ESTERÓIDES ANABÓLICOS ANDROGÊNICOS
REGISTRO DOI: 10.69849/revistaft/cs10202504270636
Lucas Caseri Câmara1
Diogo Pinto da Costa Viana2
Lucio de Sousa Monte Alto3
Abstract
Abuse of anabolic-androgenic steroids (AAS) has been widely linked to adverse health outcomes. However, establishing direct causality between AAS use and negative effects remains methodologically complex. This article presents a critical review of the literature, emphasizing the main limitations of observational studies, particularly regarding the lack of control for confounding variables. Systematic reviews and population surveys reveal that AAS users display highly heterogeneous usage patterns, often involving supratherapeutic doses, concomitant use of multiple substances (polypharmacy), and reliance on counterfeit products from the underground market. Moreover, AAS abuse rarely occurs in isolation and is frequently associated with the use of licit and illicit drugs. Users also tend to present distinct psychosocial profiles compared to non-users, including histories of trauma, body image disorders, and low self-esteem. These characteristics undermine group comparability and hinder the attribution of specific outcomes to AAS exposure. We conclude that data interpretation should be approached with caution and that future research must adopt more robust methodologies, including better user characterization and advanced strategies to control for confounders.
Keywords: anabolic steroids, testosterone, androgens.
Resumo
O uso abusivo de esteroides anabolizantes androgênicos (EAA) têm sido amplamente associado a efeitos adversos sobre a saúde. No entanto, a atribuição de causalidade direta entre EAA e desfechos negativos é metodologicamente desafiadora. Este artigo realiza uma análise crítica da literatura, destacando as principais limitações dos estudos observacionais, especialmente em relação à ausência de controle de fatores de confusão. Revisões sistemáticas e estudos populacionais demonstram que usuários de EAA apresentam padrões de uso extremamente variáveis, incluindo doses muito superiores às terapêuticas, polifarmácia com outras drogas potencialmente mais nocivas, e aquisição de produtos adulterados no mercado paralelo. Adicionalmente, o uso de EAA raramente ocorre de forma isolada, sendo comumente associado a substâncias lícitas e ilícitas. Também são frequentes antecedentes psicossociais e comportamentais que diferenciam significativamente os usuários de EAA dos não usuários, como histórico de traumas, transtornos de imagem corporal e baixa autoestima. Tais elementos comprometem a comparabilidade entre grupos e dificultam a identificação de efeitos específicos dos EAA. Conclui-se que a interpretação dos dados deve ser feita com parcimônia, e que futuras pesquisas devem incluir estratégias metodológicas mais refinadas para controle de confundidores e melhor caracterização dos usuários.
Palavras-chave: esteroides anabolizantes, testosterona, androgênios.
Introduction
The non-therapeutic and abusive use of anabolic-androgenic steroids (AAS) has been extensively associated with a wide range of adverse health effects, ranging from mild to potentially severe, as demonstrated by multiple systematic reviews and observational studies [1–3]. In a survey conducted by Parkinson AB and Evans NA (2006), involving 500 AAS users, only 0.8% of participants reported no side effects related to the use of these substances [4].
The most frequently reported adverse effects in the literature include acne and oily skin, gynecomastia, mood and behavioral disturbances, sexual dysfunction, testicular atrophy, fluid retention, insomnia, localized pain at injection sites, skin striae, increased body hair, hair loss, voice deepening, clitoral hypertrophy, hypertension, and alterations in lipid profiles and liver enzyme levels [1–4].
Although less common, more serious adverse effects have also been documented, particularly cardiovascular complications. Observational evidence suggests that prolonged and abusive AAS use may cause significant damage to the cardiovascular system, including left ventricular hypertrophy, systolic and diastolic dysfunction, and, in some cases, progression to severe heart failure [5–7].
In this context, a recent study conducted by Windfeld-Mathiasen et al. (2025) [8] followed 1,189 AAS users over approximately 11 years, comparing them to a control group of 59,450 individuals. The findings revealed a higher incidence of cardiovascular events among AAS users, with an increased risk of acute myocardial infarction, need for interventions such as angioplasty or coronary artery bypass grafting, as well as venous thromboembolism, arrhythmias, cardiomyopathy, and heart failure [8].
On the other hand, authors such as Goldman A. and Basaria S. [3] argue that the most severe outcomes associated with AAS use—particularly cardiovascular events—are predominantly reported in studies with significant methodological limitations. These include case reports and case series, retrospective case-control studies, cross-sectional research, and uncontrolled observational cohorts.
Similarly, a critical review by Fanaroff AC et al. (2020) [9] emphasizes that in the absence of robust randomized clinical trials, other forms of evidence may be insufficient to accurately estimate the true risks and benefits of such interventions. In complex topics, often marked by potential unknown or confounding factors, interpretations based solely on clinical observations or anatomical, physiological, or pharmacological knowledge may lead to biased conclusions [9].
Additionally, it is important to acknowledge that establishing clear causal relationships is hindered by the lack of distinction between therapeutic and abusive AAS use, as well as by the presence of multiple often-overlooked confounding variables. These include the use of clandestine and adulterated substances, administration of extremely high doses (sometimes exceeding therapeutic levels by more than 30-fold), prolonged and uninterrupted use, polypharmacy, concurrent abuse of other legal or illegal substances, and pre-existing health conditions [10,11].
Given this scenario, the primary objective of this manuscript is to critically and narratively explore the potential adverse effects arising from AAS abuse, while considering the methodological limitations frequently present in the current scientific literature. In a landscape shaped by biases, confounders, and a recurrent failure to differentiate between therapeutic and abusive use, it is essential to foster a more rigorous and contextualized analysis. This approach is expected to significantly contribute to the advancement of scientific research, promote more qualified debate, and provide better-informed clinical support for healthcare professionals working with AAS users.
– Underground Market:
In a review published in 2023 [12], we addressed the quality of anabolic-androgenic steroids (AAS) sourced from the international parallel market and their implications for scientific research. The analysis compiled data from several studies involving seizures and laboratory analyses of AAS in different countries, revealing adulteration rates ranging from 18% to 86%, including contamination with heavy metals and absence of the declared active ingredients. Beyond the direct health risks, the poor quality of these products represents a major confounding factor in clinical studies and case reports investigating adverse events allegedly associated with AAS use. The review highlights the importance of considering both the origin and composition of the substances analyzed in scientific research to avoid biased conclusions regarding the safety and toxicity of AAS, especially in non-controlled studies [12].
In 2024, we published a second review [13], this time focusing exclusively on the Brazilian illicit AAS market, compiling forensic data from seizures conducted across the country. The review encompassed nine studies that analyzed confiscated samples in laboratory settings, revealing significant rates of adulteration (ranging from 20% to 66.7%), with common findings including the absence of active ingredients, presence of undeclared compounds, underdosing, and the use of oily vehicles incompatible with pharmaceutical applications. These findings reinforce the alarming reality of low-quality substance consumption in Brazil, particularly in environments with high prevalence of AAS use, such as gyms and fitness centers. As observed in other countries, the review underscores the central role of such adulterations in amplifying health risks and hindering the establishment of reliable causal relationships in observational studies and adverse event reports [13].
In a pioneering study, Ferenchick (1996) [14] assessed the validity of self-reported AAS use among bodybuilders by comparing participants’ reports with urine laboratory results using gas chromatography and mass spectrometry (GC/MS). The sensitivity of self-reports was only 74%, and specificity was 82%, indicating that a significant proportion of users fail to accurately disclose their use. Furthermore, 22 out of the 23 individuals who tested positive had at least one undeclared substance in their urine, while 15 participants reported using substances that were not detected. These findings not only highlight the unreliability of self-reported data but also raise concerns about the actual composition of the products consumed, which may be adulterated or counterfeit, as previously described [12,13]. The authors advocate for the inclusion of laboratory validation in clinical studies involving AAS to minimize misclassification bias between users and non-users and improve the accuracy of causal attributions in adverse events [14].
Despite the well-documented poor quality of many of these products, the parallel market remains the main source of acquisition for most AAS users. Only 11.6% report obtaining them through medical prescriptions [4]. Observational data show that 70.8% of users purchase from online dealers, 24.2% through contacts in gyms, 18.8% import directly from other countries via mail, and 8.6% obtain the products from online pharmacies [4].
Finally, an important commentary published by Kimergård et al. (2014) [15] had already drawn attention to the methodological limitations in clinical case reports involving AAS use from illegal sources. The authors emphasized that the composition of these products is often unknown, with incorrect dosages, complete absence of the declared active ingredient, and the presence of toxic contaminants or unauthorized combinations of active substances. These products are manufactured in clandestine laboratories, without pharmaceutical quality control, substantially increasing the risk of unpredictable adverse effects. As a direct consequence, causal interpretation of clinical symptoms attributed to AAS use becomes weakened, given that many patients are unaware of the exact substances they have consumed. The authors recommend, whenever possible, conducting laboratory analyses of both the products used and biological samples from users, as a means to validate exposure and improve diagnostic accuracy in clinical cases. Self-report bias and uncertainty regarding product composition significantly compromise the scientific robustness of these reports [15].
– Dosages
According to Evans NA (1997) [16], the average supraphysiological doses used by anabolic-androgenic steroid (AAS) users hovered around 500 mg per week, with variations ranging from 250 to 3,200 mg per week—that is, between 2.5 to up to 32 times the recommended therapeutic weekly dose of approximately 100 mg. Nearly a decade after this initial publication, a substantial increase in the doses used was observed, reaching up to 6,000 mg per week, as demonstrated in a subsequent survey involving 500 users, published in 2006 [4]. In this more recent study, 59.6% of users reported taking more than 1,000 mg per week, and 12.6% reported using over 2,000 mg per week (equivalent to 10 and 20 times the therapeutic dose, respectively) [4].
In parallel, several randomized controlled trials were conducted in experimental settings, administering progressively increasing doses of testosterone to healthy young men. The administered doses ranged from subphysiological levels (25 mg per week) to six times the physiological dose (600 mg per week) [17–23]. These studies revealed a U-shaped response curve for testosterone levels. At low levels, there was a greater prevalence of symptoms associated with hypogonadism, such as physical and mental fatigue, decreased libido, sexual dysfunction, and impaired body composition. Conversely, at excessively high levels, the risk of adverse effects increased, including elevated hematocrit, increased blood pressure, hepatic strain, dyslipidemia, and behavioral changes [17–23].
Additionally, an exploratory investigation conducted by Abrahin et al. (2016), which examined AAS use patterns among Brazilian women, found that 18.7% reported using doses between 1,000 and 2,000 mg per week, while 4.2% reported doses exceeding 2,000 mg per week. These values are considered extremely high, particularly when compared to the standard male replacement dose, representing 10 and 20 times that reference level, respectively [24].
Thus, considering the U-shaped physiological response pattern of testosterone and its impact on health, it is essential that observational data analyses carefully differentiate between effects attributable to AAS per se and those that merely reflect the dose-dependent response. In other words, many of the adverse effects observed may be better explained by the quantitative intensity of exposure rather than by a qualitatively distinct pharmacological response [10,11]. This becomes especially evident when comparing with randomized controlled trials, which adhere to strict methodological criteria, such as participant screening with exclusion of comorbidities, controlled diet and training regimens, use of standardized pharmaceutical-grade compounds, absence of polypharmacy or concurrent use of other legal or illicit substances, and well-defined intervention periods (e.g., 20 weeks). Under these controlled conditions, even at significantly elevated doses, no serious adverse events were reported [10,17–23]. In contrast, the lack of control over these variables in observational studies severely compromises the validity of causal inferences.
– Polypharmacy
A systematic review conducted by Sagoe et al. (2015), which compiled data from 50 studies, raised an important concern regarding the widespread practice of polypharmacy among anabolic-androgenic steroid (AAS) users—a frequently overlooked yet potentially significant risk factor [25]. The authors emphasize that this association must be carefully considered by physicians, researchers, and public health policymakers. Concomitant use of various pharmacological classes during AAS cycles was identified, including analgesics, anti-inflammatory agents, opioids, central nervous system stimulants and depressants, diuretics, cosmetic drugs (e.g., dermatological agents), cardiovascular medications, recreational drugs, and other hormones [25].
Additionally, Parkinson and Evans [4] also mapped this pattern and categorized the medications commonly used in conjunction with AAS into four main groups: (a) accessory anabolic hormones such as growth hormone (GH), insulin, and insulin-like growth factor 1 (IGF-1); (b) stimulants including ephedrine, amphetamines, thyroid hormones, yohimbine, and dinitrophenol; (c) miscellaneous pharmaceuticals such as diuretics, muscle relaxants, and analgesics; and (d) medications aimed at mitigating side effects, including clomiphene citrate, aromatase inhibitors, tamoxifen, and human chorionic gonadotropin (HCG).
Among female users, this trend of concomitant use of additional substances has also been observed. Data from Abrahin et al. (2016) [24] reported a prevalence of 18.7% for concurrent use of diuretics, 2.1% for ephedrine, and 2.1% for clenbuterol. Moreover, over time, there has been not only an increase in AAS dosages but also in the frequency and intensity of accessory drug use. For example, in 1997, reported use rates were 12% for GH, 2% for insulin, and 2% for thyroid hormones [16]. By 2006, these figures had risen to 25%, 25%, and 45%, respectively [4].
It is important to note that many of these accessory substances, when used abusively and without medical supervision, may pose even greater risks than AAS themselves. Drugs such as insulin, thyroxine, diuretics, and stimulants carry a high potential for triggering clinical emergencies, including hypoglycemia, arrhythmias, and severe psychiatric and cardiovascular disorders [26].
Nonetheless, the side effects caused by AAS abuse are not typically perceived by users as a deterrent to continued or escalating use. Instead, many individuals resort to self-medicating with additional substances to mitigate adverse effects, rather than reducing or discontinuing AAS use [4,26].
In summary, the frequent association of multiple potentially hazardous drugs with AAS cycles significantly undermines the ability to attribute adverse effects to a single agent. In many observational studies, AAS users are treated as a homogeneous group (“AAS users”) compared to “non-users.” However, AAS use rarely occurs in isolation. This methodological limitation compromises the validity of conclusions regarding specific adverse effects if polypharmacy is not adequately accounted for in the analysis [4,16,24–26].
– Concurrent Abuse of Legal and Illegal Substances
Sagoe et al. (2015) [25] observed that the concomitant use of legal and illegal substances is a widespread phenomenon among anabolic-androgenic steroid (AAS) users. Among these substances, alcohol, tobacco, marijuana, cocaine, hallucinogens, heroin, inhalants, LSD, and methamphetamine were particularly notable. Similarly, The Anabolic 500 Survey, conducted by Ip et al. (2011) [27] through a 99-item questionnaire, identified high rates of concurrent drug use among AAS users: 47.2% reported alcohol use, 22.9% tobacco, 30.6% marijuana, 3.0% heroin, and 11.3% cocaine in the 12 months preceding the survey. Among women, data from Abrahin et al. (2016) [24] showed similar patterns, with 29.2% reporting concurrent alcohol use and 10.4% tobacco use.
Several studies suggest that in many cases, AAS abuse is preceded by a history of psychoactive substance use. The review by Sagoe et al. (2015) [25] supports this pattern, and additional data indicate that AAS users frequently have prior experience with alcohol [28,29], marijuana [28,29], opioids [26], heroin [30], and cocaine [28,29].
Furthermore, the use of performance-enhancing drugs (PEDs) is highly prevalent among these individuals. The study by Ip et al. (2011) [27], for instance, revealed that AAS users reported using an average of 11.1 different substances (up to a maximum of 29) when considering AAS and other PEDs together. Even when AAS were excluded, participants still reported the use of an average of 8.9 different substances (with a maximum of 28), a number significantly higher than that reported by non-users.
Based on these findings, it is evident that AAS abuse rarely occurs in isolation. The most common scenario involves the combined use of multiple performance-enhancing substances, legal drugs (such as alcohol, tobacco, and opioids), and illegal drugs (such as cocaine and heroin) [26–30]. It is important to note that these substances, in isolation, already pose significant health risks, which are further amplified by increased dosages and prolonged use. This complex pharmacological interaction hinders the clear attribution of causality to AAS in observed adverse events, as the effects may result from overlapping or synergistic actions among multiple substances [26].
Therefore, this factor must be carefully considered in the analysis of observational studies. However, a significant portion of the literature classifies groups merely as “AAS users” and “non-users,” overlooking the complexity of concurrent substance use. This methodological limitation compromises the validity of conclusions regarding adverse effects attributed solely to AAS, underscoring the need for greater analytical refinement in future research.
– Health and Psychosocial Background
Research on the factors that motivate the abusive use of anabolic-androgenic steroids (AAS) has revealed that such behavior is often embedded within a broader context of psychological and social vulnerability. Several studies have shown that AAS users present characteristics that distinguish them significantly from non-users, particularly regarding mental health, trauma history, and distorted body image perception [31].
A systematic review conducted by Sagoe et al. (2014) [31] demonstrated that, among AAS users, there is a higher prevalence of past anorexia, muscle dysmorphia, low self-esteem, negative body image, psychiatric disorders, drug use, and traumatic experiences such as bullying, sexual assault, and divorce. These factors appear to form a risk profile that frequently precedes the initiation of AAS use [31].
Data from the study by Ip et al. (2011) [27] support this trend: 6.1% of participants reported a history of sexual abuse, and 10.0% reported a history of physical abuse. Similarly, Gruber and Pope (1999) [32], in a study involving 75 female weightlifters, found that 13% had experienced rape, which led many of them to adopt compulsive training behaviors that eventually resulted in the use of ergogenic substances such as AAS and clenbuterol. Among these women, 70% reported using such substances with the goal of altering their bodies and enhancing physical performance [32].
In another review, Harmer (2010) [33] argued that AAS use initiated during adolescence is more closely associated with a cluster of behavioral disorders than with motivations purely related to athletic performance. Supporting this view, a Norwegian study involving 1,351 students [34] revealed, through logistic regression analysis, that alcohol use, symptoms of anxiety and depression, and illicit drug use were the main predictors of AAS use—suggesting that such behavior may be part of a broader risk pattern.
A similar study conducted by Swedish researchers (Kindlundh et al., 2001) [35], also using logistic regression analysis in a sample of 1,353 students, identified significant associations between AAS use and immigrant status, poor academic performance, low self-esteem, and the use of substances such as alcohol, sedatives, and tranquilizers. These findings reinforce the idea that, for many individuals, AAS use is not solely driven by athletic goals but rather by underlying psychological and social issues.
Furthermore, self-reported motivations among AAS users strengthen this perspective, with reasons such as “increased aggressiveness” for “personal protection” and “social acceptance” being frequently cited [27]. Notably, 8.9% of respondents reported using AAS secretly, hiding this practice even from close relatives and spouses.
Taken together, these findings make it clear that AAS users constitute a population with distinct psychosocial and clinical characteristics that markedly differentiate them from non-users. Therefore, observational analyses that compare these two groups based solely on the presence or absence of AAS use risk neglecting key confounding variables. Such omissions can compromise the interpretation of findings, particularly in outcomes related to mental health (e.g., anxiety, depression, and suicide) and risky social behaviors (e.g., aggression, violence, and substance abuse).
The complex landscape of AAS abuse, along with the main potential biases that may compromise the interpretation of adverse health outcomes (side effects and serious adverse events), is illustrated for better understanding in Figure 1 below.
Figure 1 – Main biases related to the context of AAS abuse
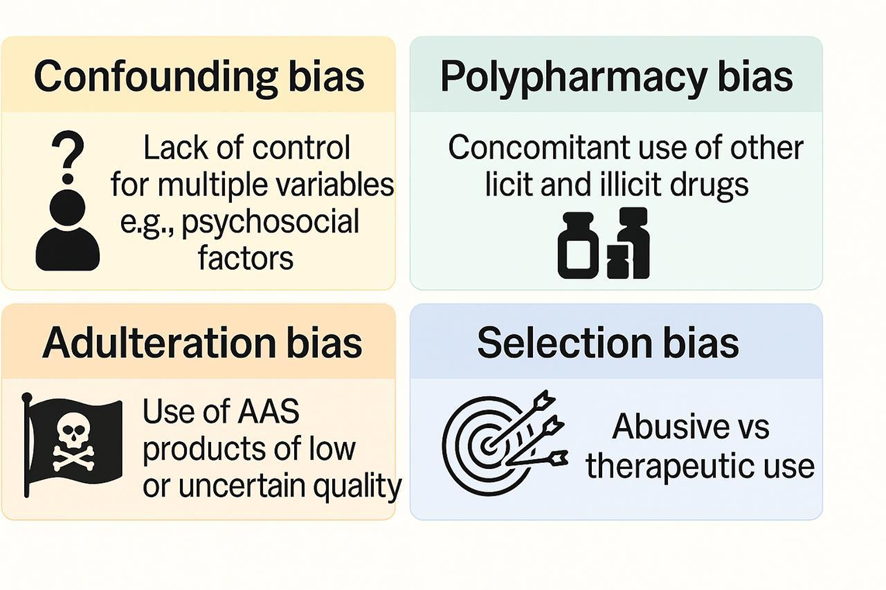
– Conclusion
The relationship between the use of anabolic-androgenic steroids (AAS) and their potential adverse health effects still presents significant methodological gaps that must be clearly acknowledged. Despite the extensive documentation of these effects in observational studies, the direct attribution of causality remains limited due to a range of factors that are frequently not adequately controlled for in the analyses. Among the most critical are the use of substances obtained from the parallel market (often adulterated and contaminated), the concomitant use of multiple drugs (i.e., polypharmacy), and the often complex clinical and psychosocial histories of AAS users.
These elements suggest that the AAS-using population possesses sufficiently distinct characteristics such that direct comparisons with non-users—commonly seen in observational studies—should be interpreted with caution. In many cases, these two groups are not truly comparable, which may lead to biased conclusions regarding the actual effects of AAS.
Therefore, it is essential that future studies progress not only in methodological design but also in the depth of user characterization, taking into account social, behavioral, and contextual variables. The use of laboratory validation, clear differentiation between patterns of use, and rigorous control for multiple confounding factors are indispensable steps for improving the quality of available evidence. Only through this broader and more careful perspective will it be possible to more accurately understand the risks associated with AAS use and to inform clinical practice and public health policies with greater responsibility.
References:
1 – Albano GD, Amico F, Cocimano G, Liberto A, Maglietta F, Esposito M, Rosi GL, Di Nunno N, Salerno M, Montana A. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare (Basel). 2021 Jan 19;9(1):97. doi: 10.3390/healthcare9010097.
2 – Frati P, Busardò FP, Cipolloni L, Dominicis ED, Fineschi V. Anabolic Androgenic Steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Curr Neuropharmacol. 2015 Jan;13(1):146-59. doi: 10.2174/1570159X13666141210225414.
3 – Goldman A, Basaria S. Adverse health effects of androgen use. Mol Cell Endocrinol. 2018 Mar 15;464:46-55. doi: 10.1016/j.mce.2017.06.009.
4 – Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006 Apr;38(4):644-51. doi: 10.1249/01.mss.0000210194.56834.5d.
5 – Baggish AL, Weiner RB, Kanayama G, Hudson JI, Picard MH, Hutter AM Jr, et al. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ Heart Fail. 2010 Jul;3(4):472-6. doi: 10.1161/CIRCHEARTFAILURE.109.931063.
6 – Abdullah R, Bjørnebekk A, Hauger LE, Hullstein IR, Edvardsen T, Haugaa KH, et al. Severe biventricular cardiomyopathy in both current and former long-term users of anabolic-androgenic steroids. Eur J Prev Cardiol. 2024 Mar 27;31(5):599-608. doi: 10.1093/eurjpc/zwad362.
7 – Rasmussen JJ, Schou M, Madsen PL, Selmer C, Johansen ML, Ulriksen PS, et al. Cardiac systolic dysfunction in past illicit users of anabolic androgenic steroids. Am Heart J. 2018 Sep;203:49-56. doi: 10.1016/j.ahj.2018.06.010.
8 – Windfeld-Mathiasen J, Heerfordt IM, Dalhoff KP, Trærup Andersen J, Andersen MA, Johansson KS, Biering-Sørensen T, Olsen FJ, Horwitz H. Cardiovascular Disease in Anabolic Androgenic Steroid Users. Circulation. 2025 Feb 13. doi: 10.1161/CIRCULATIONAHA.124.071117.
9 – Fanaroff AC, Califf RM, Harrington RA, et al. Randomized Trials Versus Common Sense and Clinical Observation: JACC Review Topic of the Week. J Am Coll Cardiol. 2020 Aug 4;76(5):580-589. doi: 10.1016/j.jacc.2020.05.069.
10 – Câmara L C. Possible Scenarios of Testosterone and Anabolic Androgenic Steroids Use in and Outside Medicine. J. Adv. Med. Med. Res. 2024, 36, 346-352. Doi: 10.9734/jammr/2024/v36i115646
11 – Câmara LC. Complexities in Assessing Health Risks of Anabolic Steroid Abuse. J. Adv. Med. Pharm. Sci. 2024, 26, 151-153. doi: 10.9734/jamps/2024/v26i12740
12 – Câmara LC. Anabolic Androgenic Steroids from Underground Market: Drug Quality and Implications for Research. Asian J. Res. Med. Pharm. Sci. 2023, 12 (3):59-64. doi.org/10.9734/ajrimps/2023/v12i3221.
13 – Câmara, L. C. Counterfeit Anabolic Steroids in Brazil: A Forensic Perspective on Quality and Risks. J. Pharm. Res. Int. 2024, 36, 130-139.
14 – Ferenchick GS. Validity of self-report in identifying anabolic steroid use among weightlifters. J Gen Intern Med. 1996 Sep;11(9):554-6. doi: 10.1007/BF02599607.
15 – Kimergård A, Breindahl T, Hindersson P, McVeigh J. The composition of anabolic steroids from the illicit market is largely unknown: implications for clinical case reports. QJM. 2014 Jul;107(7):597-8. doi: 10.1093/qjmed/hcu101.
16 – Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997 Mar;31(1):54-8. doi: 10.1136/bjsm.31.1.54. PMID: 9132214.
17 – Bhasin S, Travison TG, Storer TW, Lakshman K, Kaushik M, Mazer NA, et al. Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012 Mar 7;307(9):931-9. doi: 10.1001/jama.2012.227.
18 – Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab. 2006 Nov;91(11):4669-75. doi: 10.1210/jc.2006-0822.
19 – Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996 Jul 4;335(1):1-7. doi: 10.1056/NEJM199607043350101.
20 – Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001 Dec;281(6):E1172-81. doi: 10.1152/ajpendo.2001.281.6.E1172.
21 – Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003 Oct;88(10):1478-85. doi: 10.1210/jc.2002-021231
22 – Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002 Jan;283(1):E154-64. doi: 10.1152/ajpendo.00502.2001.
23 – Singh AB, Hsia S, Alaupovic P, Sinha-Hikim I, Woodhouse L, Buchanan TA, et al. The effects of varying doses of testosterone on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002 Aug;87(8):3624-32. doi: 10.1210/jcem.87.8.8762.
24 – Abrahin O, Félix Souza NS, de Sousa EC, Santos AM, Bahrke MS. Anabolic–androgenic steroid use among Brazilian women: an exploratory investigation. J Subst Use. 2016;22(3):246–52. doi:10.1080/14659891.2016.1179806
25 – Sagoe D, McVeigh J, Bjørnebekk A, Essilfie MS, Andreassen CS, Pallesen S. Polypharmacy among anabolic-androgenic steroid users: a descriptive metasynthesis. Subst Abuse Treat Prev Policy. 2015 Mar 15;10:12. doi: 10.1186/s13011-015-0006-5.
26 – Evans NA. Current concepts in anabolic-androgenic steroids. Am J Sports Med. 2004 Mar;32(2):534-42. doi: 10.1177/0363546503262202.
27 – Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy. 2011 Aug;31(8):757-66. doi: 10.1592/phco.31.8.757.
28 – Fudala PJ, Weinrieb RM, Calarco JS, Kampman KM, Boardman C. An evaluation of anabolic-androgenic steroid abusers over a period of 1 year: seven case studies. Ann Clin Psychiatry. 2003 Jun;15(2):121-30. doi: 10.1023/a:1024640410093.
29 – Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003 Jul 20;71(1):77-86. doi: 10.1016/s0376-8716(03)00069-3.
30 – Cornford CS, Kean J, Nash A. Anabolic-androgenic steroids and heroin use: a qualitative study exploring the connection. Int J Drug Policy. 2014 Sep;25(5):928-30. doi: 10.1016/j.drugpo.2014.06.002.
31 – Sagoe D, Andreassen CS, Pallesen S. The aetiology and trajectory of anabolic-androgenic steroid use initiation: a systematic review and synthesis of qualitative research. Subst Abuse Treat Prev Policy. 2014 Jul 2;9:27. doi: 10.1186/1747-597X-9-27.
32 – Gruber AJ, Pope HG Jr. Compulsive weight lifting and anabolic drug abuse among women rape victims. Compr Psychiatry. 1999 Jul-Aug;40(4):273-7. doi: 10.1016/s0010-440x(99)90127-x.
33 – Harmer PA. Anabolic-androgenic steroid use among young male and female athletes: is the game to blame? Br J Sports Med. 2010 Jan;44(1):26-31. doi: 10.1136/bjsm.2009.068924.
34 – Pallesen S, Jøsendal O, Johnsen BH, Larsen S, Molde H. Anabolic steroid use in high school students. Subst Use Misuse. 2006;41(13):1705-17. doi: 10.1080/10826080601006367.
35 – Kindlundh AM, Hagekull B, Isacson DG, Nyberg F. Adolescent use of anabolic-androgenic steroids and relations to self-reports of social, personality and health aspects. Eur J Public Health. 2001 Sep;11(3):322-8. doi: 10.1093/eurpub/11.3.322.
1Department of Specialization in Clinical Anabolism College of Governance, Engineering, and Education of São Paulo – FGE-SP, Brazil. Member of the Scientific Committee of the Brazilian Society of Endocrinology and Metabolism in Sports and Exercise. E-mail: lucascc_med@hotmail.com. https://orcid.org/0000-0001-6329-2897
2Chairman of the Brazilian Society of Endocrinology and Metabolism in Sports and Exercise. E-mail: pinto.vianadiogo@gmail.com. https://orcid.org/0009-0009-9245-2509
3President of the Brazilian Society of Endocrinology and Metabolism in Sports and Exercise. https://orcid.org/0009-0004-3418-8870. E-mail: lucio.endocrinology@yahoo.com
