REGISTRO DOI: 10.5281/zenodo.7916289
Celso Fernandes Batello¹
Professor Dr. Vânia d´Almeida²
ABSTRAC
This dissertation, with the support of a theoretical practical foundation, presents the scenario where is inserted the problematic in question; The Antioxidant Effect in vitro of Homeopathic Medicines Arsenicum album, Cuprum metallicum, Manganum and Zincum metallicum. It is demonstrated in the first chapters, of theoretical bibliographical substantiation, the Homeopathy and Oligotherapy as therapeutics techniques, as well as the importance of oxidation phenomena for a better comprehension of the organic phenomena, mainly in the genesis of many diseases. It is also experimentally demonstrated the homeopathic medicines antioxidant action in different dilutions in comparison with melatonin in various concentrations over the lipidic peroxidation in homogenate of mice brains measured though the malondialdehyde dosage obtained through the absorbency technique. For the analysis of the results the Kruskal-Wallys and Dunn´s Multiple Comparisons tests were realized, that revealed significant differences among the experimented groups. It was verified a greater lipidic peroxidation inhibiting effect with melatonin 1M, followed by melatonin 0.5M, Cuprum metallicum C12, Cuprum Metallicum C30, Arsenicum album C30, melatonine 0.24M, Manganum C30 and Arsenicum album C12. It was proved that the melatonin has an in vitro lipidic peroxidation inhibiting effect, and so being adopted as reference. However the new fact arises from the observation of the significant lipidic peroxidation inhibition obtained with the usage of Homeopathic medicines, sometimes with dilutions that supersed the Avogadro number, as in the cases of Cuprum metallicum C30, Arsenicum album C30 and Manganum C30 in decreasing action order. This work calls the attention for the possibility of existence of an antioxidant mechanism action of homeopathic medicine different from the know cause effect relationship.
1 INTRODUCTION
This work was idealized starting by the presupposition that, if the homeopathic medicine possesses therapeutic and preventive action, maybe, part of this action comes from an antioxidant action.
Based on Hahnneman, paragraph 20, justificative for the experimentation:
“We aren’t abees to find out this immaterial power that is found latent in the intimus essence of the medicine only with the reason’s efforts. Just by the experience (experimentation), we can note clearly the phenomena that it provokes when it acts on the healthy organism (experimentation in the healthy man)” (Pustiglione, 2001).
Analogously, experimentation will be made, but in vitro, to verify the possible antioxidant of mice’s brain, comparatively to melatonin, specifically in the lipidic peroxidation. As a measure of this antioxidant action, the malondialdehyde (MDA) will be dosed, resulting from the lipidic peroxidion reactions.
We intend to show by experience, in vitro, the homeopathic medicine action in a specific stage of the cascade of reactive species of oxygen, the lipidic perioxidation, trying to understand, through the laboratory, and by inference, the effects of these medicines in the healthy man.
We even intend this job can create possibilities for the homeopathic medicine experimentation in the other stages of the oxidative series, as well as in other experimental sectors, in order to show it is possible to perform works in Homeopathy within the scientific patterns accepted nowadays.
The work has basis on the literary works that already exists in the involved areas, emphasizing that they never presented any conflicts; on the contrary, they seemed synergic.
Although eminently experimental, this work is also based – in bibliographical research is the Medicine History.
1.1 THE MEDICINE HISTORY
The attempt of relieving and healing the pains and is confused with the humanity history itself.
However, with Hippocrates, Medicine lost its link with Philosophy and Religion, becoming science and art. According to Maffei (1978):
“Medicine is considered as art and science at the same time, being considered as a Biology branch. If we ask: when how Medicine appeared?, we can see that Medicine was born with the man, as a matter of fact, since his appearance on Earth, the man was a victim or a witness of suffering, and so, he has always observed the illnesses that came and apply them the correct medicine”.
Hippocrates, who lived from 460 to 373 BC, in the century of Pericles, epoch of well – known people like Sophocles and Euripides, Aristophaes and Pindaro, Socrates an Platon, Herodoto and Tucidedes, Rhide and Polignoto, was endowed with a high observation spirit, what could help him to gather data in a compilation called aphorisms and form the bases to the current medical knowledge. That’s why Hippocrates was called ´Medicine Father” (Maffei, 1978).
Hippocrates created the ” scientific Medicine” and with this knowledge enunciated the basic precept of cure: Similia similibus curantur ( the similar gets cured by the similar). Different from contrario contraries curantur 9 the opposite gets cured by the opposite). ( Duprat, 1982).
If Medicine weren’t something single, that includes allopathy, homeopathy and other therapeutic techniques, after enunciating, the similar principle, Hippocrates would be acclaimed as the ” Homeopathy Father” .
The cure by opposite was defended by Galeno. By his method it is used the ” anti”, that is, facing a fever it is used an anti-fever, facing a grub ( vermin), an anti – vermin and a bacterid an antibiotic.
The cure by the similar was defined by hippocrates, when he enunciated the following aphorism:
“The illness is produced by the similar, and by the similar that produced it (…) the patient becomes healthy again. This way provokes painful urine retention. That doesn’t exist, heals the painful urine retention that exists; the cough, like the painful urine retention is caused and healed by same agent.” ( Duprat, 1982).
Hippocrates, given a practical example, mentions a case of cure of cholera with Veratrum album, that produces in the healthy man a violent gastroenteritis with tendency to algidity, similar to what happens in the choleric attack ( Duprat, 1982).
Some people think Hippocrates didn’t even exist, however there are proofs that he was born in Cós, 460 B.c. and died in 337 BC (Maffei, 1978).
The tradition informs that Hippocrates descended from Esculapius, by fatherhood and from Hercules by motherhood. Among his ancestors there were some kings and 3 famous doctors: Prodicus de Cos, Hippocrates his and I father Heraclitus, who taught him the first scientific notions. This way he was Heraclitus and Phenavitas´s son, or Praxitea, from the Asclepiades family, that were performing the Medicine for 18 generations. Hippocrates was undoubtedly, a wise man, like Socrates and others (Maffei, 1978).
1.1.1 HOMEOPATHY HISTORY
Homeopathy is the therapeutic method based on the application of a pharmacological law called Similarity law or similitude principle (Tetau, 1980).
This law was enunciated by Hahnneman, Homeopathy creator, doctor that was born in Meissen( Saxen), 1755. Samuel Frederich Hahneman in his medicinal substances virtues, affirm: “To radically cure certain chronic diseases, one must look for medicines that commonly provokes in the human organism an Analogous disease and the most analogous as possible”.(Tetau, 1980).
1.1.2 HAHNEMANN, “HOMEOPATHY FATHER”
Hahneman, considered as the Homeopathy Father, studied Medicine in Leipzig, and died, in Paris when he was 88 years old. His mortal remains are in Pere Lachaise Cemetery, in Paris, whose city is proved of keeping the remains of this immortal humanist (Castro, 1980).
1.1.3 HOMEOPATHY HISTORY IN BRAZIL
Homeopathy was introduced in Brazil on November, 21, 1840, by the Frenchman Benois Jules Mure (Castro, 1980).
In 1918, through the decree 3530, September, 25, the Hahnemanneano Institute of Brazil was recognized as an entity of public utility, in the article that says: “Besides the medicine supplied by the official schools, the Homeopathic Clinic will be performed by the professionals qualified by the Hahnemanneano Institute” (Castro, 1980).
In 1952, by the decree 1552, July 8, 1952, the teaching of Homeopathic Pharmacotechnique becomes a must in all the Pharmacy Colleges in Brazil (Castro, 1980).
1.1.4 HOMEOPATHY AND ALLOPATHY
As has been reported, Hippocrates enunciated 2 principles of cure: Contrario contraries curantur and smilia similibus curantur and similia similibus curantur, that is the cure by the contrary, held mainly by Galeno, and the cure by the similar adopted by Samuel Hahnnemann, “Homeopathy Father” .
Hahnemann, in his gifted life realized that if one diluted the substance even more, the cure would be faster, soft and lasting. That’s why
Homeopathy commends the use of medicines that cure by the similar and diluted doses that are potencialized though suction processes, characterizing minimum doses (Kent, 1980).
In the homeopathic medicine administration all the individual’s aspects are taken into account, that is, the physical, psychical and mental, trying to choose that medicine that acts properly. The doctor that in the first cases had already chosen a medicine that approaches the homeopathic specific will be able to verify the security of the chosen medicine in the next cases, or else he will be able to find out the proper one (Hahnemann, 1984).
1.2 MEDICINES
1.2.1 HOMEOPATHIC MEDICINE
The homeopathic medicine can be divided in polychrestus and minor medicines, according to its pathogenesis that is the capacity of provoking symptoms in a healthy individual. The polycherestus are those that produce some stronger and more accentuated pathogenesis symptoms than the minor medicines. The term polychrestus is formed by 2 Greek words: much and powerful ( Tetau, 1980).
The elaboration of the homeopathic medicine obeys to established rules in the country with the decree nº 57477-66 that dispose about the manipulation, prescription, industrialization and sale of the homeopathic industrialized products determination nº 1180, august 1997, according to the solution nº23, December 6, 1999 (Brazilian Homeopathic Pharmacopoeia)
Let’s use examples to better illustrate the method:
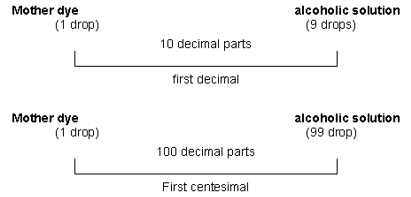
Acting like this, consecutively searing by the first ones, we can get the subsequent decimal and centesimal potencies.
Once we’ve performed such dilutions, the homeopathic medicine must pass through a process called dinamization, that is, to such the bottle tapping on its bottom with the palms (suction) or else, to perform the same procedure in a special device assigned for this purpose.
The term dilution must be reserved to the series of successive operations described thoroughly in the several Organon editions, and that allows decreasing more and more the quantity of medicinal substances (Demarque, 1981).
1.3 HOMEOPATHIC MEDICINE ORIGIN
The homeopathic medicine comes from the 3 reigns that exist in the nature: animal, mineral and vegetal.
As an example from the animal reign we have the snake poison (Lachesis trigonogaster); from the mineral reign, the copper (cuprum metallicum), the Gold (aurum metallicum) and the mercury (mercurius solubillis), and finally from the vegetal, the belladonna extracted from the plant Atropa belladonna. (Duprat, 1982).
1.4 THE MEDICINE TESTED IN THE HEALTHY MAN
One of the Homeopathy’s principle is the experimentation in the healthy man, what defines that the same medicine that will Leal the sick one by the similarity Law (Tetau, 1980).
This way, the practice of Homeopathy implies:
a) The experimentation of different substances administrated in the healthy man. In this sense we can say that the Homeopathy is an experimental method. The whole of the observed signals during this experimentation, made obviously in sub toxic doses, gets the name of pathogenesis. Some of pathogenesis comes from substances unproved of toxic things (calcium carbonate, sea salt, silica). They point out the notion of particular sensibility of certain individuals.
b) Medicine in weak dose, and even very weak, rising up many times beyond the number of Avogadro ( Tetau, 1980).
Hahnnemann experimented several medicines, and after a rigid observation, he catalogued the symptoms and signals produced by these substances in his look Pure Medical Matter (Kent, 1980)
1.5 THE ACTION OF THE HOMEOPATHIC MEDICINE
According to the Similatarity Law, the homeopathic medicine, tested in the healthy man, causes characteristic physical and mental symptoms, and they were registered by Hahnemann in his already mentioned book Pure Medical Matter.
It’s know that the choice of the homeopathic medicine is made by a process of exclusion, unique and particular, in what the patient’s symptoms must coincide as much as possible with the medicine. That’s why it’s said that one is the mirror of the other (Tetau, 1980).
The adage from Hippocrates similia similibus curantur shows a precept that more clearly can be defined like this: the medical substance able to establish in the healthy organism a set of analogous disturbances that exist in the sick organism.
The healing action of the homeopathic medicine is revealed through a mechanism that can be analyzed according to two points of view: pharmaco-dynamic, that reveals the duality of the action of all medical substance according to the doses applied, and biological, that interferes in the specificity of the organic defense (Duprat, 1982).
The duality of action of all medicine has been known for many years, and, not mentioning Heinnemann, we can find it in the conclusions of several biologists, such as:
Claude Bernard: “Every substance that, in small doses, excites the qualities or functions of an anatomic element, in high doses, repeals them”(Duprat, 1982).
Brown-Séquard: “The moderate excitement of a nervous element provokes exaltation of the functions that depend on it directly or by reflex; a strong excitement can affect the same functions” (Duprat, 1982).
Hugo Schultz: “Every excitement provokes in a cell increasing or decreasing of its physiological function, according to the strong or weak intensity of the excitement” (Duprat, 1982).
Huchard: “It’s enough to say that I held, long time ago, only two therapeutic laws: the one from the treatment and cure of a great number of morbid states with medicine that produce analogous symptoms to the diseases, and the one from the prescribed medicine in minimum doses, once it’s true, like Pecholier said, from Montpelier, that in a single medicine there are several medicines, according to different doses” (Duprat, 1982).
Rudolf Arndt, according to the Fundamental Biology Law states: ” The little increase it the strong ones subdue it, the exaggerated ones, abolish it. This law is from the ” shaking Law”, by Plunger, that fixes the action of the weak, medium and strong electric current” (Duprat, 1982).
Let’s appeal to some common examples, in order to make concret these constant pharmaco-dymanics.
“The opium, in strong doses, is a brain spinal depressor and it produces a comatose sleep with muscular relaxation, in small doses it stimulates the intellectual, nervous and muscular activity, and it acts as a great excitant to keep person awaken” (Duprat, 1982)
Talking about the arsenic, in its classical treaty of therapeutics, Manquat says: ” The action of the arsenic in the real gloves can be destructive, however, on the teeth is seems to act differently. Actually in minimum doses, this medicine stimulates the production of red globes, and in strong doses it’s destructive” (Duprat, 1982).
In short: “the effect of the strong does, which Hehnnemann called primitive, and other authors called active, talks about the action natural to the medical substance, its toxic virtue and a coercive action, of denominated secondary or reactive, refers to action natural to the organism, its defense reaction, its liberation from the toxic threat of the medical substances” (Duprat, 1982).
The IpecaCuãnha in strong doses is a vomitory, in weak doses, diluted and strengthened, homeopathic, becomes one of the medicines for nauseas and vomits. This way there is pharmacological inversion to a certain dosage” (Tetau, 1980).
1.6 HOMEOPHATIC MEDICINE EXPERIMENTATION
This research is based on the in vitro experimentation of the following homeopathic medicines: Zincum metallicum, Cuprum metallicum, Arsenicum Album and Manganum.
1.6.1 ZINC
The zinc is a transition metal that performs a lot of organic functions, being one of the main metals for the brain. It takes part in the insulin formation as well as acts in the performance of almost a hundred of enzymes of the system denominated zinc-finger, controlling and activating a big quantity of hormones” (Halliwell and Gutteredge, 1989).
The zinc is abundantly found in the prostate gland secretion, in the seminal liquid, what suggests the cause and effect relation between the zinc fault and the benign prostate gland hyperplasia” (Handler, 1990).
Plentiful in the nature, but combined with sulphur or silica the Zincum metallicum can be found in France, England, India and Australia.
Even Hahnnemann who gave it us proper in therapeutics, it has hardly ever been used in Medicine. Thanks to the homeopaths works they could get good results from this remedy (Lathoud, 1980).
1.6.2 ZINCUM METALLICUM
Zinc, used as a homeopathic medicine, gets the Latin name Zincum metallicum.
Zinc is a solid laminated metal, ductile, white bluish. It’s fragile when it’s dry and heated until 200ºC, that’s why it’s necessary to keep it at an intermediary temperature, when it’s exposed to humidity it gets covered by a thin hydrocarbon cover, that adheres strongly to the metal avoiding its oxidation. Heated until it’s live red, burn to the air like beautiful green flame and comes to oxide of zinc, Zn O. (Lathoud, 1980).
Zinc acts on the set of sympathetic nervous system and spinal brain. However its action falls back particularly on the thorax plexus and on the big trunks and nervous branches that are distributed by the locomotor system, that controls the movements and sensibility (Lathoud, 1980).
Zinc’s performance occurs in the motive part as well as in the sensorial, above all in the nervous system. What iron is for blood, zinc is for the nervous system ( Lathoud, 1980).
1.6.3 COPPER
Copper is a transition metal that can be found in every tissue, however in bigger quantities in the brain and liver.
Copper acts as a co – factor in several enzymatic processes, as catalytic converter in the synthesis of the hemoglobin, in the conversion of tyrosine in melatonin of the thyroids hormones T3 and T4, in the structural protection of the myelin sheath and in the elastin and collagen synthesis. It also integrates the enzyme superoxide desmutases, in the cytochrome oxidize in the tyrosinase and in the beta hydroxilase dopamine (Torti, 1988).
1.6.4 CUPRUM METALLICUM
Copper used as a homeopathic medicine gets the Latin name Cuprum metallicum.
Copper is metal with a characteristic red color, very malleable, ductile and tenacious. At fresh air it’s covered by green from hydrocarbon ate (grayish green). It’s found in the nature mainly in the state of pyrite of copper and iron sulphate, often associated to antimony, silver, lead and arsenic sulphates, and also in the state of oxide and hydrocarbonate. It is also present in the most of vegetal and animal food (Lathoud, 1980).
Copper acts on the spinal medulla and the sympathetic nervous system selectively, performing leading influence in the whole body. It also acts, in the motor and sensitive enervations and in the trophic enervation affecting the nutrition deep and directly ( Lathoud, 1980).
1.6.5 ARSENIC
The arsenic is a toxic metal, whose contamination sources are combustible oils, insecticides and tinctures. It’s eliminated through urine.
From the toxic metals, arsenic is one of the least dangerous. However, the inorganic arsenics and the dangerous. However, the inorganic arsenics and the trivalent forms are more toxic. Systemically, when they are absorbed orally they can provoke vasodilatation in massive doses, the arsenic can provoke deleteriores effects in the cardio-circulatory system, like destruction of the capillaries and arterioles, as well as myocardic necroses. In the gastro-intestinal treatment it can provoke serious lesions like severe hemorrhages and alterations of the cellular proliferation (Lathoud, 1980).
The application in Homeopathy happened from the arsenic toxicological observations on the human being, being used in these circumstances, of course, dilested, when these effects are not observed.
1.6.6 ARSENICUM ALBUM
The arsenic used as a homeopathic medicine gets the Latin name Arsenicum album.
In general, the Arsenicum album is used as therapeutic agent of great potency and diffusion. It has an immense field of action: ” it embraces the whole organism, and because of its elective localization on the sympathetic nervous system it is meaningfully affected. We can say that it irradiates to all the organic systems”. (Espanet apud Lathoud, 1980).
In a single word, the action of this unique polychrestus is indefinite since the benign stages (weak irritation), until the extreme cachexia (complete, chronic action). ( Lathoud, 1980).
1.6.7 MANGANESE
The manganese is a transition metal, that takes part in the molecule of mitochondrial superoxide dismutasis, from where one can infer its relevant importance in the endogenous mechanism of stress oxidative control and lipidic peroxidation (Torti, 1988).
The manganese acts as a co-factor in the synthesis of biotin, acetylcholine, cholesterol, thyroid hormones, thiamin, vitamin C and prothorombin as well as acts as an activator of the peptidase, the arginase and also in the glucose metabolism and in the absorption and transportation of copper. (Hendler, 1990).
1.6.8 MANGANUM
The manganese used as a homeopathic medicine gets the Latin name Manganum.
The manganum, white-grayish metal, hard, breakable, inalterable when exposed to the environment, to normal temperature it is presented under a petroleum cover. To hot air, it’s lightly revested by an oxide cover. It disintegrates slowly in cold water, faster in ebullition. Under pulverization this decomposition is very fast, even under normal temperature. It can be found in many minerals in the state of oxide silicate, phosphates, sulphurus, and sometimes in plants ashes, in bones and also blood (Lathoud, 1980).
In Homeopathy it is used pulverized metallic manganese and with it the 3 first dinamizations are prepared by hahnemanneana trituration (Lathoud, 1980).
Manganese or Manganum aceticum, acts deeply on the nervous system, the skin and the bones, followed by a serious anemia state and intense debility.
1.7 REACTIVE OXYGEN SPECIES – ROS
1.7.1 THE ORIGEN OF OXYGEN
The molecules of diatomic oxygen in the earth atmosphere are the biggest ones to provoke reactions on the living cells. Thus, it’s appropriate to start with some comments about, the oxygen, only later we will have some considerations about the nature and definition of free radicals.
Except for those organisms that are specially adapted to live under anaerobic conditions, all the animals and plants need oxygen for an efficient production of energy (Halliwell and Gutteridge, 1989).
The oxygen emergency must have been followed by the ozone layer appearance (O3) in the high atmosphere, and the absorption of the damage effects of the ultraviolet the evolution by the ozone, layer probably allowed the evolution of the most complex earth organism (Halliwell and Gutteridge, 1989).
1.7.2 THE OXYGEN METHABOLISM
The oxygen is the element that, in the periodic classification of the chemical elements, belongs to the family 6A, whose atomic number and atomic mass are 8 and 16 respectively, and it possesses 8 electrons distributed in its orbitary layers.
Normally, around 95 to 98% form the oxygen absorbed by the aerobic organisms is reduced, forming water in the breathing chain though the electrons transportation in the mitochondria as well as in the endoplasmic reticulum, where the enzymatic system cytochome, in the process of oxidative phosphoroclastic reaction proceeds the tetravalent reduction of O2 by the cytochrome oxidase system, putting up simultaneously 4 electrons to the oxygen, that it is directly reduced to the water:
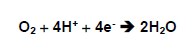
The sources that give the cations of hydrogen and the electrons to the reaction are, basically, the NADH, the FADH and the ubiquinone or co-enzyme Q ( Halliwell and Gutteridge, 1989).
However, as already referred, from 2 to 5% of O2 is reduced only 1 electron, and this one is going to occupy one of the external orbitals, at the same time that the other keeps not matched, producing intermediaries highly reactive, called Reactive Oxygen Especies, that sometimes constitute the free radicals. This way, the first reactive toxical specie of oxygen, the superoxide, like in the scheme:
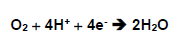
1.7.3 FREE RADICAL
Free radical is any spice able of independent existance and which contains one or more electrons unmatched, that is, electrons present individualy in atomic or molecular orbitals (Halliwell and Gutteridge, 1985).
Na unmatched electron can join to isolated atoms (hydrogen, metallic ions) or even to molecules (sugar, proteins, lipids, DNA) what comes to be a process of biologic relevance (Slater, 1984 – Halliwell, 1987).
On the other hand the free radicals have already been related to several human illnesses and they take part as fundamental components in many of them, what shows us how big is the oxidative damage caused by them (Halliwell and Gutteridge, 1985).
1.7.4 HYDROGEN PEROXIDE
Another way of ROS is the hydrogen peroxide.
The hydrogen peroxide, although it’s not a specie very reactive, it is na agent able to inactivate enzymes, mainly by oxidation of essential thiol groups. Its bigger oxidative power, however, is indirect, as the generator of the hydroxil radical (HO∙ ) and by the interaction with the superoxide radical (O2∙ –). In this case it’s a powerful oxidative, and in enough concentrations, it can kill can cells, and in the presence of iron its toxicity can increase from 10 to 100 times (Eaton, 1991).
There are 2 kinds of enzymes that remove the hydrogen peroxide. They are the catalase and the peroxidase glutathione ( Halliwell & Gutteridge, 1989).
Under the peroxidase glutathione action, the hydrogen peroxide reacts with the reduced glutathione, oxidate, and forms 2 molecules of water and oxidated glutathione.

Glutathione redutase
The oxidated glutathione is once again reduced (regenerated) by the action of the redutase glutathione enzyme. We can notice that for the perfect working of the peroxidase glutathione, the presence of sellenium is fundamental, and for the redutase glutathione action there must be vitamin B2.
Under the catalase action, there is also the formation of water, besides the molecular oxygen, So we have the following reaction:
Catalase
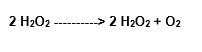
The HO can be generated in the cells by exposition to the ionizant radiations, from other species of oxygen, as well as by the Fenton and Haber-Weiss Reactions, mediated by metallic ions.
1.7.5 FENTON REACTION
The mixture of hydrogen peroxide and Fe2+ reacts to many organic molecules, as was firstly observed by Fentom, in 1984. The reactivity is due to the hydroxil radical formation (Halliwell and Gutteridge, 1989).
Intermediary complex
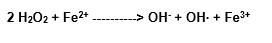
Traits of Fe3+ are available to react again with the H2O2, although such a reaction is very slow in physiological pH:
Intermediary complex]
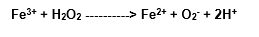
The simple mixture of the with the hydrogen peroxide can ceirtainly form, in the biological system, under some conditions, a range of oxidative reactions (Halliwell and Gutteridge, 1989).
1.7.6 HABER-WEISS REACTION
This reaction occurs in the presence of iron or copper salts, like the following:
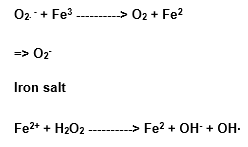
Or copper
The ferrous complexes are able of the hydroxil radical formation (OH), as they present a low molecular mass, besides the O2∙ – and the H2O2 facility of liberating iron ions active in a catalytic way, coming from proteins. Thus the increase of the O2∙ – and the H2O2 generation can creat conditions to the formation of the OH. (Halliwell and Gutteridge, 1985).
1.8 OTHER PHYSIOLOGICAL CONDITIONS
The reactive species can also be produced by the utilization of the diet compounds (Ames, 1989).
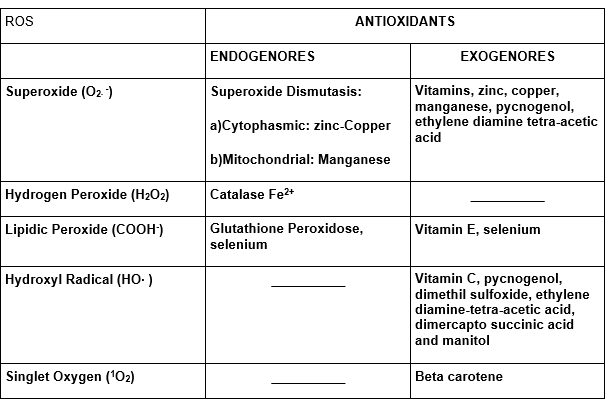
Following we have the table with the main reactive oxygen species (Sies, 1991).
TABLE 1: Reactive Oxygen Species – ROS
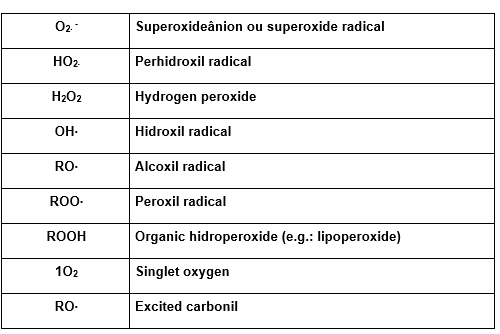
1.9 ROS AND ANTIOXIDANT SYSTEMS
Not all the ROS have systems that make them inactive. For some ROS there are endogenous desativator systems, while for the others there are external antioxidants, or even both.
The external antioxidants are also called scanvengers or ROS sweepers (Halliwell and Gutteridge, 1989).
Following there is the ROS table related to their correspondent antioxidants.
TABLE 2: Reactive Oxygen Species and Antioxidants
1.10 ROS FORMATION
The ROS can by very toxic when excessive, either by elevated production or by organism difficulty in neutralizing them. They are formed starting by the oxigen, what is a paradox, because at the same time that it generates and support the life, on the other hand it can be lethal (Halliwell and Gutteridge, 1989).
Firstly the superoxide radical (O2∙ –) is formed, which can be dismuted in hydrogen peroxide (H2O2) or even through catalytic action, by the acting, of the superoxide dismutasis enzyme (SOD).
In the organism there are two main SODs, one of them cytoplasmic, that is the CuZnSOD, and the other mitochondrial, that is the MnSOD, this on containing manganese and that one containing Copper-Zinc in the same molecule.
The importance of the SOD can be shown by the fact of being the most abundant enzyme of the organism, as well as it also the fifth most abundant protein in the same organism. (Halliwell and Gutteridge, 1989).
The following graphic shows how the ROS formation occurs.
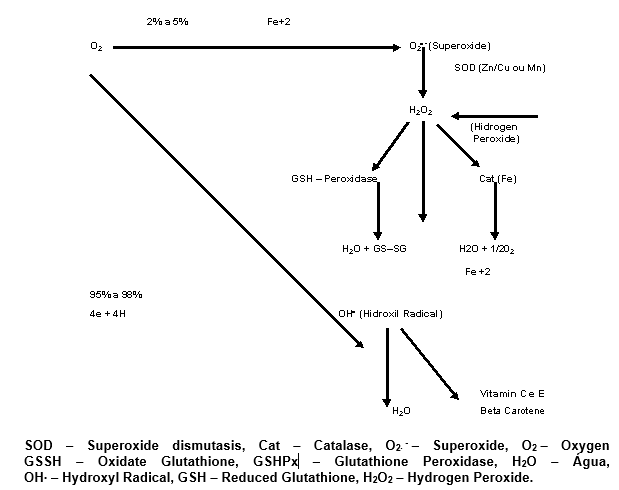
1.11 ROS ACTIONS IN THE BIOLOGICAL SYSTEMS
The man is probably the result of evolutionary processes of unicellular and anaerobic organisms. The terrestrial environment gained more complex organisms only layer development, which allowed the absorption of part ultraviolet solar radiation, limitative factor for life, that for a long time, was confined to the aquatic enviroment. Whith the ultraviolet radiation reduction, which causes damages to the living beings, the terrestrial environment became compatible to life, unchairing the acceleration of the evolutionary process (Halliwell and Gutteridge, 1989).
As a result of these changes, the man remained with remanescent of his anaerobic system, that’s why the oxygen one breaths – and that is so important to maintain the life in different circumstances – can be the cause of death or diseases development, as it is one of the most important generators of free radicals inside the organism (Halliwell and Gutteridge, 1989).
The ROS, as they react to the majority of the organism molecules, they are able to interfere in the biological processes, causing several diseases, mutations, oldness, among other alterations (Halliwell and Gutteridge, 1985).
1.11.1 ISCHEMIA AND REPERFUSION
The ischemia comes from the initial obstrution of the blood flux, for exemple, due to the arterial clamping for a certain period of time, what provokes tissue suffering by hypo-oxigenation. With the unclamping, the tissue reperfusion occurs, determining the brusque arreval of oxygen and nutriens to the tissue, with a meaninful encrease of free radicals, extreme important phenomenon, mainly during the first 60 to 90 seconds, because it damages the reperfused area, provoking micro-heart attacks (Haliwell and Gutteridge, 1989).
Tissues that suffered hypoxia or ischemia survive for variable periods, depending on the nature of the tissue involved.
It the period of ischemia or hypoxia is short, the tissue damage can be minimized with the re-introduction of nutrients. The re-introduction of O2 in the ischemia or long tissue hypoxia – can cause – aditional tissue injury (re-oxygenation injury) which is partiatly mediated by oxygen radicals (Halliwell and Gutteridge, 1989).
The ischemia and reperfusion may occur in the cardiac surgeries, where the re-oxigenation can cause serious arrhythmias with extense lesions due to the oxygen paradoxal shock, which causes ATP depletion during the ischemia, forming hypoxanthine or xanthine, that work like a substrate to the xanthine oxidase enzyme (XO), precursor form the uric acid, that acts like na antioxidant in these cases.
During the re-oxigenation, through the reperfusion, the free radicals production occur with its deleterious effects, what makes the ischemia even worse (Halliwell and Gutteridge, 1985).
The xanthine oxidase is produced from the xanthine dehidrogenase in the presence of the ion Ca2+, generating free radicals that will damage the tessues, that’s why the use of antioxidants like the SOD or the manitol is commended before the arterial inclamping.
1.11.2 NEUROLOGICAL DISEASES
It has been observed that cranium-encephalic traumatisms cause cerebral injury or spinal medulla. The lead can provoke degeneration that involves reactions with free radicals, like the lipoperoxidation.
Cerebral ischemia or hypoxia, followed be reperfusion, must also estimulate the lipoperoxidation (Halliwell and Gutteridge, 1989).
The brain contans 3 important characteristics:
1. It is very rich in polyunsaturated fatty acids that act like substrate to the lipidic peroxide formation;
2. It is free iron richest organ, which acts forming the radical hydroxyl;
3. It’s formed by cells that do not reproduce themselves, the neurons, that reach the maturity around 30 years old and, later, they will be lost at about 10.000 to 100.000 a day.
Another mechanism that contributes to the cerebral injury after the hypoxia ischemia is the non production of exciting neurotransmitters animoacids, like the glutamate or aspartate ( Halliwell and Gutteridge, 1985).
In the Parkinson disease, most part of the works has shown there are free radicals increase, mainly in the brain’s nucleus that will produce L – dopa and are mainly cytotoxins and neurotoxins, that cat at this level increasing the superoxide production (Halliwell and Guteridge. 1985).
The Alzheimer disease, senile insanity, presents some proper characteristics, like: neurofibrillar tangles, ghost neurons, lipoferous deposit (pigment formed by the malondialdehyde connected to the protein), matched filaments, amyloidosis (the amyloidosis protein precursor, APP, is a potent free radicals generator), increase in the activity of the neuritic plaques and oldness of some parts of the brain like: raphe neclei, hyppocampus and locus ceruleus (Halliwell and Gutteridge, 1985).
The aluminium is the most abudant metal of the terrestrial crust and we are constantly exposed to it. The presence of high aluminium concentrations in the brain of patients with alzheimer suggests this is one of the causes of the causes of encephalopathy present in the disease (Halliwell and Gutteridge, 1985).
Two of the pathological characteristics of the Alzheimer dementia, firstly observed by Alois alzheimer in 1906, are the presence of senile plaques and neurofibrillar tangle in the brain. The aluminium action must contribute to the neurotoxic proprieties, as we know the brain is sensitive to the free radicals reactions (Halliwell and Gutteridge, 1989).
1.11.3 LIPIDIC PEROXIDATION
The lipidic peroxidation is the process through which the ROS attack the polyunsaturated fatty acids of the membranes phospholipids of the cells, desintegrating them and allowing, this way, these species entrance in the intracellular structures.
The phospholipases, activated by the toxic species, desintegrates the phopholipids, liberating the non saturated fatty acids (Halliwell and Gutteridge, 1989, resulting in the following deleterious actions of the lipidic peroxides:
a) cellular membranes rupture (NA/k and Ca/Mg bombs)
b) DNA mutations – deoxyribonuclei acid
c) Unsaturated lipids oxidation
d) Chemical residues formation like the malondialdehyde
e) Components engagment of the extracellular matrix, proteoglycans, collagen and elastin
The lipidic peroxides possess na action power higher than the other primary toxic species of O2 (O2∙ –, H2O2, OH∙ , O2), reaching further targets easily.
The lipoperoxidation must also have a very important role in the cellular proliferation, especially in tumoral cells. Some authors suggest that the lipoperoxidation products are involved in the cellular division control. On the other hand the lipid peroxidation is related to the tumoral increase (Gonzalez, 1992).
1.12 ANTIOXIDANT MECHANISMS
The oxygen is a paradox in the planet, because it is essential to live as well as it can cause injures to the organism (Halliwell and Gutteridge, 1985).
The antioxidant agents can’t distinguish between the reactive oxygen species that have a physiological role and those that are causing damage. Because of this, their action can, in some not be profitable to the organism. However it is with the balance between the oxidant and antioxidant species that the organism, will be able to obtain the conditions to a better performance of its functions, considering that a disturbance in this balance can result in a range of pathological processes (Bast et al, 1991).
In general the following strategies have to be followed with the intention of increasing the efficiency of the OA.
Oxidative Stress
1.13 ANTIOXIDANT DEFENSE
The antioxidants that represent the organisms defense against the reactive oxygen species are divided in two main kinds, the non enzymatic and the enzymatic.
1.14 NON-ENZYMATIC ANTIOXIDANTS
Some essential nutrients can attack directly the oxygen radicals. The vitamin E (alfa-tocopherol) is the biggest liposoluble anti-oxidant present in all the cellular membranes, and therefore, acts in the protection against the lipoperoxidation (Kay et al., 1986). It can react directly with a variety of oxiradicals, like the superoxide, the hydroxil, etc, and also with the singlet oxygen ( Machlin and Bendich, 1987).
The vitamin E was firsty related in 1922, in the USA, by Evaris and Bishop, who demonstrated it is liposoluble and also essential factor for the normal reproduction in mice. The purification of this factor revealed it is compound from the tocopherol family. Four tocopherols are known, yet the alfa-tocopherol is the most improtant biologically and the terms alfa-tocopherol and vitamin E (Halliwell and Gutteridge, 1989).
First of all the inactive alfa-tocopherol reacts with the singlet and could, this way, protect the membrane against this specie.(Halliwell and Gutteridge, 1989).
During its antioxidant action like chain – breaking (destroying the lipoperoxidation chain) in the membranes the alfa-tocopherol is consumed and converted in form of radical (Halliwell and Gutteridge, 1989).
The vitamin E can also protect against the peroxidation, modifying the membrans structure (Halliwell and Gutteridge, 1989).
The vitamin E, situated near the cytocrome P-450 in the membrane phospholipid, sweeps the free radicals formed in the cytocrome P-450. Then, the vitamin C reduces the radical tocopheril. In a recent study held at Tufts University, it was proved the powerful imuno-stimulant action of the vitamin C. (Halliwell and Gutteridge, 1989).
The vitamin E, called the vision vitamin was discovered at about 2.000 years, when the Greeks verified that the animals’liver had something that cured some eyes affections, that’s why the name retinol, due to its importance for the vision. It was the first vitamin to be cataloqued, therefore the name A. (Halliwell and Gutteridge, 1989).
The retinol is essential in the human diet and it is usually known as vitamin A, one of the liposoluble vitamins (Halliwell and Gutteridge, 1989).
The carotenoids, mainly the beta-carotene can work as vitamin A precursors. They are absorved by the human bowels and must also actuate like antioxidants. They have a double role, they decrease the singlet oxygen formation in vivo, and help to remove those already formed. (Halliwell and Gutteridge, 1989).
The vitamin A has little antioxidant action and it is unable to act on the singlet oxygen, but its precursor, the beta carotene, is the most efficient linking of this reactive form of oxygen found in the nature and it can act like antioxidant, the beta carotene, a pigment present in all plants, can be found in cellular membranes, including in the lipossomes (Machlin and Bendch, 1987).
The pure ascorbic aced is soled, white, crystalline and very soluble in water. Plants and animals can synthesize it, except the humans, primates and cavies that can’t synthesze it and need to obtain it from the diet (Halliwell and Gutteridge, 1989).
The ascorbic acid is necessary in vivo like cofactor of several enzymes, and the most well-known are the proline hydroxylase and the lysine hydroxylase, involved in the collagen bio synthesis. The ascorbate deficiency in the human diet causes the scurvy . The most impressive chemical propriety of the ascorbate is its alility to act like reducer agent (electrons donor). (Halliwell and Gutteridge, 1989).
The vitamin C (ascorbic acid), is hydrosoluble and also acts against the free radicals and the singlet oxygen. The ascorbic acid also takes part of the regeneration of the vitamin E antioxidant and reduced form (Halliwell and Gutteridge, 1985).
The vitamins A, C and E, can also be found in high concentrations in the plasma, in the adrenal glands, in the brain, liver, and in the blood cells among other regions (Porta, 1988).
The is also a range of other non-enzymatic antioxidants that take part in the defense against the reactive oxygen species in the biological systems like, for exemple, the ubiquinone, the ceruloplasmin, the uric acid, the taurine, the flavonoids and other fenolic compounds of vegetal origin (Halliwell 1990, Cutler, 1991, Sies, 1991).
The ubiquimone is used a lot to improve the cardiac function in the cases of congestive cardiac insufficiency, myocardium ischemia, angina pectoris and arterial hypertension. It has been used in the multiple sclerose and the Alzheimer diease. It also acts in the mellitus diabetes, the periodontic diseases and muscular dystrophies, as well as in the imunological system disfunctions (Halliwell and Gutteridge, 1985).
The glutathione (GSH) is a cellular health marker and its fall indicates oxidant lesion. Its deficit causes decreasing in the resistance to drugs and radiations, the capacity of tumor reversion and the ascorbate syntheses in animals (Halliwell and Gutteridge, 1985).
The glutathione is a tripeptide composed by non-essential aminoacids, describe as an important antioxidant agent (Ames, 1983, Halliwell and Gutteridge, 1985).
As we could infer from the chemistry of the oxygen, the univalent reaction, generates O2.-. The main escape system is observed thorgh the complex NADH- Coenzyme Q redutase and from the reduced forms of the coenzyme Q10. (Halliwell and Gutteridge, 1989).
The human neutrophil and several other tissues (nervous tissue, for example) are taurine-rich. There are several propositions saying the taurine has a biological role and acts as an antioxidant (Halliwell and Gutteridge, 1989).
1.14.1 THE MELATONIN
The melatonin is a well-known antioxidant, produced by the pineal and gland and its main hormone was discovered by herner in 1958. It is also produced in other tissues, like retinal and large intestine. (Guyton, 1973).
The pineal gland is the first endocrine gland that is formed in the embrional phase. Its works like a “biological clock, when it secretes the melatonin at night, united to other neuropeptides (Maffei, 1978).
The melatonin secretion is ten times bigger at night than during the day. This concentration reduces as the time passes, reaching the top in the adolescence. In the elderly it corresponds to half comparing to the yoyngsters (Smith and Tier, 1990).
Chemically, the melatonin is nominated by 5 acetyl – 5- methoxy-serotonin and it is derivative from the essential aminoacid tryptophan, found in proteinous food like grains, seeds and vegetables.
Recently, the melatonin was described as part of the immine functions of the organisms and as a mighty antiioxidant. Performing the antioxidant function, the melatonin seems to unchain a substantial protection against the free radicals which are generated in a variety of experimental situations, including the lesion for ischemia reperfusion. That’s why it has been used therapeutically in surgeries and transplants (Reiter and Maaestroni, 1999).
1.14.2 MINERALS
Beside these, there are several essential nutrients from mineral origin, that take part in the antioxidant process in association with enzymes. They are: zinc, copper, manganese, selenium and iron (Halliwell and Gutteridge, 1985).
1.14.3 ENZYMATIC ANTIOXIDANTS
Antioxidants are any substances that when they are present in minor concentrations, compared to those oxidizable substrata, meaninfully delay or inhibit this substratum oxidation and they can act in different levels of the oxidative sequence (Halliwell and Gutteridge).
In 1954, Gershaman and Gilbert proposed that most of the harmful effects caused by the high oxygen concentrations in live organisms could be attributed to the free radicals formation. However this idea didn’t raise many researcher’s interest until 1968 with the discovery of na enzyme that is specific for the catalytic removal of na oxygen radical (MCCord and Fridovich, 1969). This enzyme called superoxide dismutases, along with catalase and glutathione peroxidase are the main antioxidant defenses that act in the superior organisms (Halliwell and Gutteridge, 1989).
1.14.4 ENZYMES
The enzymes: superoxide dismutase, catalase and glutathione peroxidase represent the main endogenous defense of the organism.
1.14.4.1 SUPEROXIDE DISMUTASE
It’s possible that the superoxide dismutase (SOD) be a substance with real anti-aging effects, and it can act positively over all the degenerative processes (Hendler, 1990).
The superoxide dismutase (SOD) has a fundamental role in the organism defense against the reactive oxygen species because it acts removing the superoxide. Before it was discovered, the SOD had already been described by some authors as a protein that contains copper, but they hadn’t attributed to it any catalytic activity (Halliwell and Gutteridge, 1985).
After McCord work however (1969), with the determination of its function in the superoxide radical dismutation (O2∙ –), its role was established and even today, although there are many researches with this enzyme, no other substrate was described, showing its specificity to the superoxide (Halliwell and Gutteridge, 1985).
In 1938, T. Monn and D. Keilin in England, described a blue-green protein, isolated from the bovine flood that also contained copper and called it hemocuprein. In 1953, a similar protein was isolated from the horse’s liver and called hepathocuprein. Other proteins of this type were isolated, like the brain – cupreine from the brain. In 1970, it was discovered that the proteins from the erytrocyte contain zinc as well as copper. No enzymatic function was detected in any of these proteins, so it was suggested that they served like metals deposit. However, in 1969, Jim Cord and J.Fridovich’s work, in the USA, showed that the protein from the evythrocyte can remove in a catalytic way the superoxide radical, and thus this function was identifed as the superoxide dismutase enzyme (Halliwell and Gutteridge, 1985).
There are different kinds of SOD, depending on the metal that acts like a co-factor in its catalytic site, but all of them act basically according to the same reaction described by McCord and Fridovich in 1969:
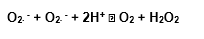
1.14.4.1.1 SUPEROXIDE DISMUTASE COPPER-ZINC DEPENDENT
The form that contains copper and zinc, called superoxide dismutase copper-zinc dependent (CuZnSOD), is very stable and seems to be present in practically all the eukaryotic cells (plants or animals). (Halliwell and Guttiridge, 1985).
On the other hand, in prokaryotic cells like seaweeds and bacteria, the catalytic activity related to CuZnSOD seems to be restricted to the symbiosis of these organisms with eukaryotes where the enzyme is present (Fridovich, 1978. Halliwell and Gutteridge, 1985).
The eukaryotic CuZnSOD also called SOD A, has a molecular weight of 32000 and it is formed by two identical proteinous sub-unities, with on atom of copper and one of zinc in each one. It’s the cytoplasmic form of the SOD, and its properties have been really resisting to the evolutive changes, beingle able to distinguish the enzyme gotten from fungi, plants, fowls and mammals easily (Fredovich, 1977).
The copper takes part in the dismutation reaction passing alternately by oxidation and reduction, like the exemple:
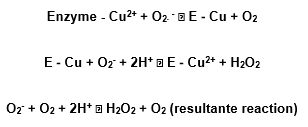
At last, another action mechanism is also possible in what the first O2– does not reduce the copper ion, but forms a complex with it.The Zn does not work in the catalytic site, but is appears to stabilize the enzyme. This conclusion was obtained from experiences in which the metals were removed from the active sites and replaced in others, alone or in groups (Halliwell and Gutteridge, 1985).
1.14.4.1.2 SUPEROXIDE DISMUTASE DEPENDENT ON THE MANGANESE (MnSOD).
The superoxide dismutase dependent the manganese (MnSOD) is a pink protein, whose molecular weigh is 40.000 and it contains manganese in the active sites. Its activity decreases in alkaline pH. It is not inhibited by the cyanide neither by di-etil-di-hydrocarbonate. It is destroyed by the chloroform + ethanol (it does not survive to the typical methods of the purification to the CuZnSOD). The MnSOD activity related to the CuZnSOD depends on the tissue and species vhere they act. The Mn removal form the active sites causes catalytic activity loss, and it cannot be replaced by any transition ion, because it loses its functional activity. The aminoacid sequences of all the MnSOD, in all the species, are allke, and they are not related to the CuZnSOD (Halliwell and Gutteridge, 1989).
The superoxide dismutese manganese dependent (MnSOD), found in bacterial like E-coli and Streptococus mutans (Mc Cord et al., 1971, Fridovich, 1978), does not seem to have any relation with the CuZnSOD included in the eukaryotes cytoplasm, except by its catalytic activity.
There is also the mitochondrial superoxide dismutase or SOD B in human tissues (Beckman et al., 1973), that is similar to the prokaryotes MnSOD but it has four sub-unities instead of two, and it has a molecular weight of about 80.000, having also a manganese atom per sub-unit. The mitochondrial form of the SOD is much more similar to the prokaryotes MnSOD than to the CuZnSOD (Fridovich, 1978).
1.14.4.1.3 EXTRA CELLULAR SUPEROXIDE DISMUTASE
Finally, there is one more form of SOD in human tissues different from the others already described. This enzyme has molecular weight of 135.000 and it is composed of four iqual sub-unities more covalently connected. Each molecule seems to contain four copper atoms, and iron or manganese are not found in the enzyme. Because it is present mainly in extracellular fluids like the plasma it was nominated estracellular SOD (ECSID). Its activity is not very significant compared to the other superoxide dismutase forms (Markeund, 1982).
1.14.4.1.4 PARTICULARITIES
Related to the catalytic activity of the SOD different forms, we can assure the CuZnSOD is reversibly inhibited by the cyanide and by H2O2 in concentrations over 10 uM (Fridovich, 1978). It can also suffer inactivation by the exposition to the superoxide radical, while the MnSOD is not inactivated in the same conditions (Senet et al, 1981).
The CuZnSOD form is more resisting to temperature variations and to denaturing by substances like guanidine chloride, dedecyl sodium sulphate, or urea (Halliwell and Gutteridge, 1985). It’s also the most resisting to pH variantions (Fredovich, 1978).
The immunological tests have been more used to the protein quantificatton or CuZnSOD and MnSOD. As these proteins are very different, the antibodies do not make crossed reactions. The tests are limited in human tissues to they serve as master lines. They are better evaluated in the liver, because there is a bigger concentration of SOD (Halliwell and Gutteridge, 1989).
1.14.4.2 GLUTATHIONE PEROXIDASE
The glutathione peroxidase enzyme (GPX) was discovered by Mills in 1959, in mammals’tissues. It is not present in plants or bacteria, although it can be found in some seaweeds and fungi (Halliwell and Gutteridge, 1985).
The substrate to the GPx is the tripeptide glutathione, found in the most of animals, plants and even in some bacteria. The enzyme catalyses the reduced glutathione oxidation (GSH) to oxidanted glutathione (GSSG) using the hydrogen peroxide:
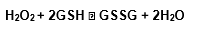
The maintenance of GSH levels occurs by the action of the glutathione redutase enzyme (GSSG), once again in its reduced form (Cohen and Hochstain, 1963; Paglia and Valentine, 1967):
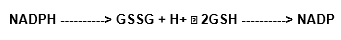
Although the GPx is specific for glutathione as hydrogen donor, it can accept other peroxides besides the H2O2 (Beutler et al, 1974; Halliwell adn Gutteridge, 1985), in reaction that can be shown like:
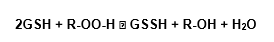
The animal cells have two kinds of glutathione peroxidase, and one of them is selenium dependent while the other is not.
The first kind is able to reduce any organic hydroperoxide , besides H2O2. This form has a molecular weight of 81.000, it is a tetrameric protein and it has one atom of selenium in each sub-unit (Forstron et al, 1978; Meera Khan et al, 1984; c Bridge et al, 1988).
The relation between the GPx activity and the selenium supply in vivo has been object of lots of clinical and experimental investigation. In human beings, the enzyme genic regulation in lineage of myeloid cells (HL-60) showed to happen pos-transcriptionally being controlled by the selenium availabelity (Chada et al, 1989).
The second kind, which is not selenium dependent, has a molecular weight of 35.000, it is dimeric and it is able to reduce any organic hydroperoxide, except the H2O2 (Meera Khan et al, 1984; Ciriolo et al, 1991).
The GPx has high activity in the liver and erythrocites, while in the brain, heart and lungs its activity is moderate, and in the muscle it is very reduced (Cohen and Hochstein, 1963).
The GPx finds high activity in the liver, moderate activity in the heart, lungs and brain, and low activity in the muscles (Halliwell and Gutteridge, 1989).
In the most of animals, the selenium dependent enzyme is responsible for the major part of the GPx varies a lot among the different species as well as from tissue in the same specie (Mannervik, 1985).
In mice, the GPx distribution has been widely studied and, in hepatocites the GPx selenium dependent is located mainly in the cytosol and in the metochondrial matrix (Mannervik, 1985),
The good pH for the GPx is next to 8.0 but the enzyme keeps active with high values. Its activity is minimum in pH lower than 6.0 (Mills, 1959; Paglia and Valentine, 1967).
1.14.4.3 CATALASE
It is an enzyme that is present in most of the aerobic cells, and in animals it is found chiefly in the liver, kidneys and erythocytes. However organs like brain, heart and skeleton muscle contain small quantities of the enzyme (Halliwell and Gutteridge, 1985; Masters and Crane, 1990).
In mammals, like rats and mice, the most part of the enzyme catalytic activity happens in the peroxisomes, and only a small part has a cytoplasmic or reticular origin (Master and Crane, 1990; Percy 1984).
In 1818, Thénard had already observed that the hydrogen peroxide was decomposed by animal tissues, with oxygen discharge. After 83 years, Loew established that this effect was due to a specific enzyme called catalase. Warbing in 1923, suggested that the catalase has iron atoms. In 1990, Geili and Helestrom found evidence that its prostetic group was the humatin (Percy, 1984).
The catalase mechanism of action can be synthesized this way, according to chance ( 1952 a and b):

If it is not neutralized, the H2O2 interacts with iron cations (or copper), originating the ion hydroxil (inert) and the free radical hydroxil (active).
Although the knowledge about the catalase mechanism of action in vitro is old, until 1971 the information about its biological role in vivo was still considered undevoloped (Chance and Oshino, 1971).
The most important nutrients that are coadjutant are the iron and the tocopherols (vitamin E), which are distributed in the cellular membraine, in the hydrophobic phase (Halliwell and Gutteridge, 1985).
After the identification of the primary intermediary of associated enzymes with the hydrogen peroxide in the fractions of rat’s liver peroxisomes, rich in mitochondrias, it was possible to establish the function of this enzyme-substrate complex also in the biological systems, considering that this action is considerably peroxidative (Chance and Oshino, 1971).
The catalase avoids the metahemoglobin accumulation and it decomposes the hydrogen peroxide, a toxic product from the metabolism, in water and molecular oxygen (Chance and Oshino, 1971; Wieacker et al, 1980; Gaetani et al, 1989).
Beside its role as reactive oxygen specie and therefore, causer of oxidative stress, the H2O2 surplus causes the hemoglobin oxidation and, thus, diminution of the oxygen concentrations, what can cause infections, ulcer and even necrosis (Wieacker et al, 1980).
Despite the reactions involving the catalase have been studied since the last century, the exact mechanism of action of the enzyme still causes argument and its biological role keeps being researcheg. It is peroxide elimination, the catalase also acts in the oxidation of electrons donors, like ethanol, methanol, phenols, DOPA, epinephrine, when the H2O2 has low concentrations (Percy, 1984).
The catalase enzyme has molecular weight of 240.000, and when it is purified, it presents 4 subunits, each of them with a grouping (Fe III- protoporfirin) connected to its active site (Keilen and Hartree, 1945; Wieacker et al, 1980).
The molecule dissociation in its sub-units causes loss of its catalytic activity. It happens easily when the enzymes is stored with freezing or infreezing, or by exposition to acids or bases (Keilen and Hartree, 1945; Chance, 1952 a; Chance, 1952b; Percy, 1984).
The catalytic activity of this enzyme can be, inhibited by superoxide, azide, hydrogen cyanide (HCN), but it is not inhibited by other cyanide ions (CNT) (Chance, 1952b). The most used inhibitor, however, it is the triazol amine that acts over the compound I, when the presence of the hydrogen peroxide is necessary so that the inhibition happens (Halliwell and Gutteridge, 1985).
1.14.4.3.1 INTERACTION
Recently, it was described the catalase inhibition by the glutathione and by other compounds with thiol grouping, like the dethiotreitol (DTT), and the reduced forms of these compounds have a bigger unhibitory action than the oxidative (Sun and Oberley, 1989).
Relating to the pH we can observe a reduction of the enzyme activity under Ph 4.0. In the level of 4.0 to 8.5 the catalase activity remains constant, and above this level it reduces again (Chance, 1952).
Experiments have been carried out to evaluate the competition between the catalase and gluthathione peroxidase enzymes in erythrocytes.
According to some authors the catalase is the enzyme that makes the conversion of high H2O2 concentrations into water and oxygen; when the hydrogen peroxide is present in low concentrations (normal physiological conditions), however, the glutathione peroxidase is in charge of transforming it into water (Cohen and Hockstein, 1963, Sinet at al, 1975; Halliwell and Gutteridge, 1985).
Gaetani et al (1989) assured that the catalase and the GPx perform the same activity in human erythrocytes, and that the decreasing of one of them wouldn’t cause deleterious effects.
Trying to establish the importance of the erythocytary catalase, Scott et al (1991) used normal and acatalasemic human cells to verify if in fact, the enzyme has a secondary role in the hydrogen peroxide metabolism, like several authors assured (Cohen and Hochstein, 1963; Sinet et al, 1975, Halliwell and Gutteridge, 1985). According to them, the use of acatalasemic cells proved the great importance of the catalase which can be the first in defense of the H2O2, once the GSH increasing or reduction (which would allow the elevation of the GPx activity) didn’t alter the general antioxidant activity in the normal or acatalasmic cells.
Both systems seem to have advantages and disadvantages to the organism while the GPx is more efficient (it has more affinity by the substrate), multi-functional (it reduces free H2O2 and also other peroxides), slow (limited by the GSH recycling) and metabolically expensive, the CAT has low affinity by the substrate but it is extremely fast (Eaton, 1991). Thus the CAT must protect the calls of large H2O2 quantities and the low endogenous levels must be in charge of GPx, along, with the enzymatic system GSH dependent (Eaton, 1991).
1.15 OLIGOELEMENTS
Forsen, in 1897, elaborated the first scientif definition of oligoelements: “oligoelements are chemical elements which are present in the living matter in concentrations equal or inferior to 0,01% of the dry corporal weight of the human organism”(Torti, 1988).
Forsen’s definition is undoubtedly very important, fowever only from the quantitative point of view, because it doesn’t refer to the metabolic and biochemical processes of the oligoelements (Torti, 1988).
1.15.1 QUANTITATIVE DIFFERENCE
Ahead Forsen’s definition, it was verified the existence of the essential oligoelements, that is, indispensable to life.
To be considered as essential, an oligoelement must have the following characteristics:
1. it must be present in all healthy tissue of all living organism;
2. its tissue concentration must be relatively constant;
3. its lack induces to physiological and structural alterations of many kends;
4. it prevents or corrects the morbid affections, caused by its lack ( Torti, 1988).
It is interesting to emphasize the similarity or analogy between the oligoelement and the vitamins, mainly concerning to itens 1 and 2, because the lack of vitamins also induces to functional and structural alterations, and they can be prevented or corrected with the proper administration.
Because of this similarity sometimes the oligoelements are called “inorganic vitamins”( Torti, 1988)
1.15.2 CO–FACTORS
A big quantily of enzymes depends on or requires na extra component, so that its enzymatic proteins can perform their catalytic functions. This extra component gets the generic denomination of co-factor (Torti, 1988). The co-factors have a didatic division in prostetic groups, coenzymes and metallic activities.
A prostetic group can be considered a co-factor tightly connected to the enzymatic protein as we can observe with the porfiniric nucleous of hemoprotein peroxidase or the flavine-adenine-dinucleotide which is strongly connected to the succinic desidrogenasis (Mitropoulos, 1995). The metallic activators group is represented by metallic cations monovalent or bivalent like K+, Mn++, Mg2+, Ca2+ or Zn2+, which are indispensable to the activities of a large groups of enzymes. Their connections can be loose or tightly connected to an enzymatic protein, probably by quelation with aminated or caboxilated phenolic groups. However, the Fe2+ connected to a group or porfirin and the CO2, connected to the vitaminie complex B12, are classified in the same group where the porfirin and the vitamin B12 take part (Torti, 1988
1.15.3 ENZYMATIC PROCESS
The oligoelements are inorganic ions and it is known that a big part of the enzymes cotain them, or at least, need them to act like that (Torti, 1988).
When na enzyme has na oligoelement in its molecule it is called metaloenzyme (Torti, 1988).
Refering to the oligoelements mechanism in the enzymatic context it is believet that:
1. some oligoelements such as copper and iron perform a catalytic function. Their presence in the enzyme proteinous part stimulates its functioning;
2. some oligoelements act, like metalic ions, as union factor between the enzyme active principle with the substrate that makes it active;
3. some oligoelements act as a powerful center of ectronic attraction, getting important oxireduction reactions (Maffei, 1978).
Considering what was said, this work entends to show, by experiments, the homeophatic medicine action starting by the assertive that the Homeopathy can use the resources and the same procedures conventionally adopted by the science.
An aspect of reasonably relevance is the possibility of demonstrating the action of a mechanism (antioxidant) differently from the usual ponderous doses. This effect is possible by the characteristic dilution of the hompeopathic medicine, that many times is above the Avogadro number.
Another thing to emphasize is that this work can bring the perspective of new studies and scientific researches particulary about the several phases of the oxidative chain, as we have limited studies about it. Besides, broadening the knowledge about the oxidative chain functioning would surely bring a larger dominion over the homeopathic medicine use as a whole, making possible to broad its therapeutic efficiency to other medical areas too.
The homeophatic medicine choice of cuprum metallicum, zincum metallicum and manganum occurred because it is known that this elements takes part in the superoxide dismutase. The Zn-Cu superoxide dismutase acts in the mitochondria. The homeopathic medicine Arsenicum album was chosen because its pathogenese fills the characteristics of stressed individual, what could also result in cellular oxidative stress. In this meaning it was entended to establish such medicines relation among them and with the melatonin, with the dilutions 6CH, 12CH and 30 CH.
By the Avogadro number, starting by the 12ª centesimal there is no matter anymore. The 6ª and 12ª centesimals have only traits from the medicine, what brings out the existence of other antioxidant medicines, besides the ones known decurrent of the minimum doses action highly diluted.
Well, if the homeopathic medicine acts in vitro, not obeying the Law of similars, it is much possible that, under such a flaw, its action is even bigger, because it supposably interferes in the homeostasis.
At last the work points to the possibility of the interchange among the different areas of the medical-scientific community, in na approach that proposes to rescue the medicine singleness and to contemplate the human health globally.
2 GOALS
This work has a main goal: to investigate the antioxidant activity of the homeopathic medicines Arsenicum album, cuprum metallicum, zincum metallicum and manganese, comparatively to the melatonin.
This way, there was following procedure:
1. determination of the inhibitory activity of lipidic peroxidation in rats brain homogenate using the melatonin as positive pattern.
2. determination of the inhibitory activity of lipidic peroxidation in rats brain homogenate using the homeopathic medicines Arsenicum album, cuprum metallicum, zincum metallicum and manganum in the 6ª, 12ª and 30ª centesimals.
3. Comparison between the antioxidant action of the different melatonin concentrations and the homeopathic medicines cuprum metallicum, zincum metallicum, manganum and Arsenicum album.
4. results evaluation for a better comprehension of the homeophatic medicine mechanism of action.
3 MATERIAL AND METHODS
3.1 MATERIALS
3.1.1 ANIMALS
Albinic rats from the wistar race were used, from the biothery of the Psychobiology Departament in São Paulo Federal University, where they were kept to the temperature of 23 ± 2ºC, with clear-dark cicle of 12 hours and free access to water and food (Pellets Labina)® .
The animals were kept in plastic cages and each cage had 6 animals. They were divided in groups of 6 male young rats, sacrified by beheading. All the animals brains were collected for malondialdehyde dosages to lipoperoxidation measures.
3.1.2 REAGENTS AND EQUIPAMENTS
The following reagents used in this work come from SIGMA Chemical Company (Saint Louis, Mo, USA): thiobarbituric acid (TBA), trichloroacetic acid (TCA).
The other reagents, sodium phosphate (Na3PO4) and sodium chloride (NaCl) come from MERCK (Rio de Janeiro, Brazil).
The experiments were held using the following apparatuses:
a) Hitashi spectrophotometer;
b) Sowall centrifuge RC-5B ( Refrigerated Superspeed), by Du Pont Instruments;
c) Potter tissues homogenizer nº B25357 in teflon, by Thomas, Philadelphia, USA;
d) Micronal balance, Model B1600;
e) Mettler balance, model AJ100;
f) Ph gage metrohm Heresau, model E520;
g) Double-boiler Evlab, model EV015;
h) Tube agitator Phoenix, model AT56;
i) Automatic pipettes Gilson-pipetman (p-20, P-200 and P-1000);
j)Double-boiler with agitation Dubnoff FANEN, model 145
3.2 METHODS
3.2.1 RESEARCH METHODOLOGY
The research made use of the homeopathic medicine Arsenicum album, cuprum metallicum, zincum metallicum and manganum, in order to check their antioxidant action in rats brain homogenate, comparatively to the melatonin, adopted as a positive patern because it is a mighty antioxidant in vitro.
The experimentation made use of homeopathic medicine regulated in the country, according to the decree nº 575477-66, wich is in charge of the manipulation, prescription, industrialization and product’s sale used in Homeopathy, determination nº 1180 form August 1997, under regulamentation according to nº 23 from December 6, 1999 of the National Agency of Sanitary Vigilance ( Brazilian Homeopathic Pharmacopocia).
The medicines were supplied by Argentum drugstore, by the pharmacist Edson Godoy.
The sample is composed by rats’brain from 3 young rats for each experiment.
The brain perfusion procedure was made with Sodium Phosphate plug 40 mm, NaCl 140 mm and pH 7,4 and it was homogenized with 3 volumes of the same plug (w:v). The next step was to centrifuge to 3000 rpm for 10 minutes.
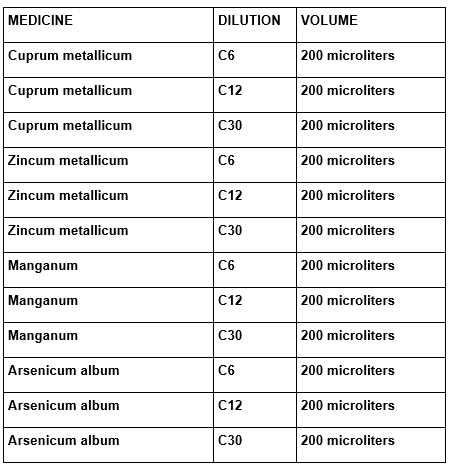
The super natant was diluted in the proportion of 1 to 4 volumes of plug and incubate to 37ºC, for one hour with agitation of different melatonin concentrations or homeopathic medicine according to the following tables:
TABLE 3: Melatonin Concentrations used in the essay
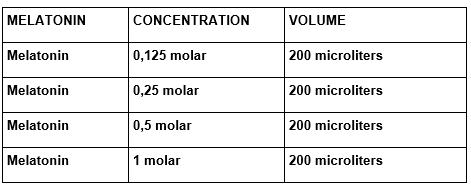
TABLE 4: Medicines used and their respective potencies
Following, 1ml of the mixture was taken out from all incubated bottles and 1 ml of TCA 5% was added, what is equal to the MDA BASAL ( time of the reaction). At the end of the incubation it was added 1 ml of super-natant with 1 ml of TCA 5%. All the tubes were centrifuged for 15 minutes to 10.000 rpm. Finally, it was added 1 ml of TBA 0,67% to the super natant. The tubes were closed and next put into double-boiler to 100ºC for 20 minutes; right after they were put into the ice for 20 minutes.
The reading was made in espectrophotometer in 535 mm, using distiled water, like white.
All the dosages were used in duplicate.
The estimate of the malon dial dehyde levels (MDA) produced was held considering the coefficient of the molar extinction ( = 4,747), using the following equation:
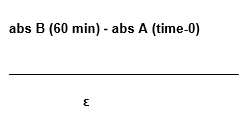
Where:
A = time 0 (no incubation)
abs = absorbance
B = average of the values gotten to the reaction duplicates (incubation for 60 minutes)
3.2.2 STATISTIC METHOD
For the analysis of the results gotten, tests non parametric were used, considering the nature of valves distribution of the variables studied, or the variability of the measures studied. The kruskal-wallis (ANOVA) and Dumn’s multiple comparison’s tests were applied.
It was established in p ≤ 0,05 the demanding level for the hypothesis of nullity
4 RESULTS
With the values gotten by the experiments, it was held the analyses of variation for non parametric data proposed by kruskal-walles, whose result showed there are significant differences among the several experimental groups, P< 0,0001 (KW=93,90).
TABLE 5: Values gotten in Dunn’s multiple comparisons analysis
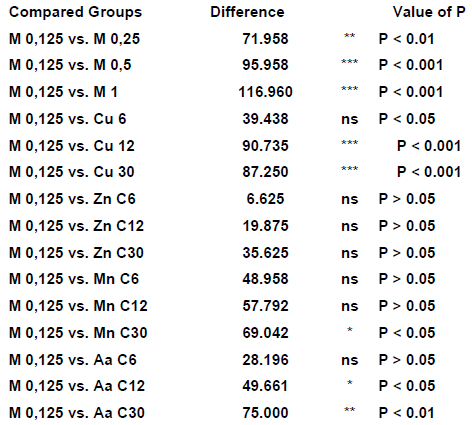


TABLE 6: Significance level of the different, Experimentation groups gotten by the analyses of Dunn’s multiple comparisons:
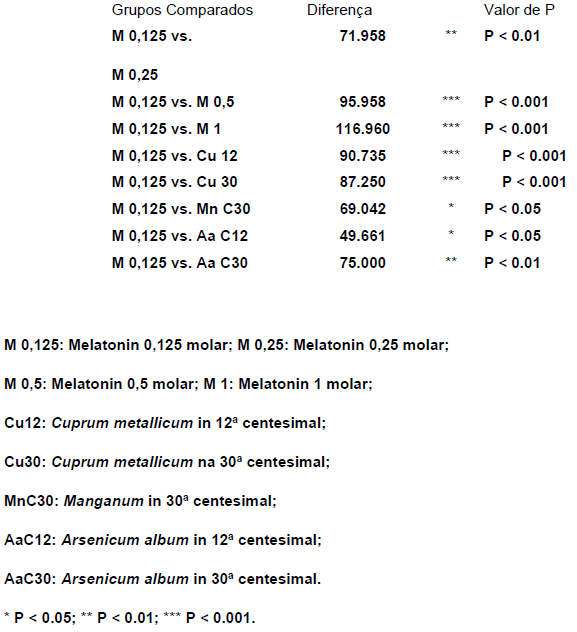
The melatonin used in the concentration 0,125M didn’t produce peroxidation inhibition, being adopted this way as parameter for the other experimentations, either of the melatonin itself, in different concentrations, or the homeopathic medicine in several dilutions.
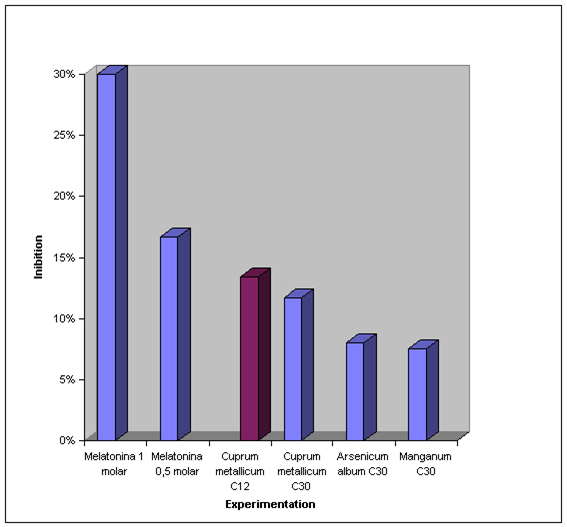
We can notice that the bigger inhibitive effect of the lipidic peroxidation happened to the melatonin 1 molar (30%), coming next the melatonin 0,5 molar (16,7%), the cuprum metallicum C12 (13,4%), cuprum metallicum C30 (11,7%), Arsenicum album C30 and melatonin 0,25 molar (8%) and manganum C30 (7,5%).
The other analysed groups didn’t present significative differences.
It was observed that, among the homeopathic medicine experimented, the cuprum metallicum C12 was the one that presented greater lipidic peroxidation inhibition grade.
It was noticied a significant lipidic peroxidation inhibition with the use of homeopathic medicine above the Avogrado number, such as cuprum metallicum C30 and Arsenicum album C30.
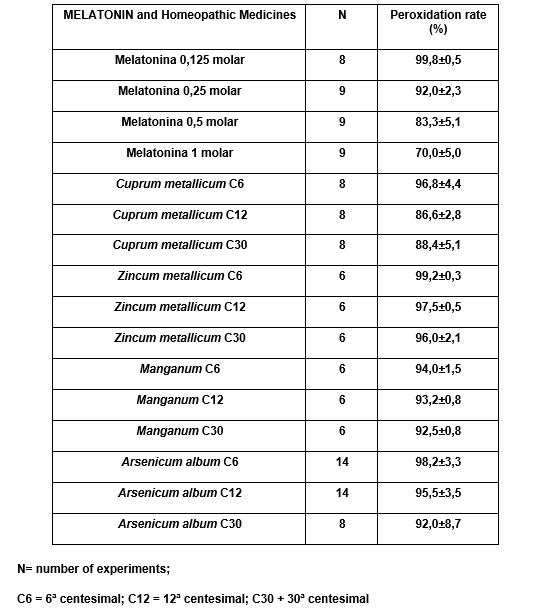
In the table 7 we present the averages of lipidic peroxidation porcentages gotten for each essay and in the graphic 1 the levels of inhibition in this process are present, which are gotten for the melatonin and the homeopathic medicine, and that present significant differences.
TABLE 7: % of Lipidic Peroxidation in rat’s brain homogenate trying the homeopathic medicine Cuprum Metallicum, Zincum Metallicum, Manganum and Arsenicum album, comparatively to the melatonin M 0,125 molar:
GRAPHIC 1: Lipoperoxidation Inhibition Percentage
EXPERIMENTS
5 DISCUSSION
The research focused on the homeopathic medicine use in order to measure the probable antioxidant effect of this medicine in rat’s brain homogenate in the lipidic peroxidation comparatively to the melatonin, because the melatonin has proved to have antioxidant action in vitro.
Hahnnemann, in chapter IV, which talks about the pharmaco-dynamic of the substances, mentions: “We are not able to find out this immaterial force that is latent in the close essence of the medicine just with the reazon’s efforts. Just by the experience (experimentation) we can clearly notice the phenomena it provokes when it acts in a heallhy organism” (Pustiglione, 2001).
As the homeopathic medicine experimentation always occurs in vitro, following the same reasoning, we made the experimentation in vitro.
The homeopathic medicine is widely used in the whole planet, despite of no knowing its real action mechanism, once such mechanisms are stell no perfectly explained.
The experimentation in vitro can complement the knowlegde of the homeopathic medicine pathogenese itself, as well as its action in the several organic alterations.
This effect reproduction opens perspectives to other essays that allow a better comprehension of the homeopathic medicine action in vivo.
In this work it was possible to verify the antioxidant action of the homeopathic medicine: cuprum metallicum, arsenicum album, zincum metallicum and manganum, comparatively to the melatonin, inhibiting the lipoperoxidation over the young male rat’s brain homogenate.
The melatonin presented significant action in the lipidic peroxidation inhibition, that’s why it severed use a comparative pattern of reference in the use of the homeopathic medicine mentioned.
These homeopathic medicines choice was due to th fact of them being part of the mitochondrial and cytoplasmic superoxide dismutase. The arsenicum album was chosen because it is na important polychrestus whose pathogenese fits to the profile of the stressed individual psychically, that eventually can cause cellular oxidative stress. These elements passed through trituration process before being diluted, what means they suffered succussion.
The work showed the homeopathic medicines cuprum metallicum, manganum and arsenicum album are significant as inhibitor in vitro of the lipidic peroxidation in rat brain homogenate, comparatively to the melatonin in substanteal dose through the melondialdehyde dosage.
Through the experiments analysis it was noticed, having the melatonin 0,125 M as a reference, less inhibition of the lipidic peroxidation. There was more inhibition of the lipoperoxidation with the use of the homeopathic medicines cuprum metallicum, manganum and arsenicum album in the 6ª, 12ª and 30ª centesimals. However, the same effect was not observed to the Zincum metallicum.
The significance gotten through the cuprum metallicum and Manganum experimentation can be explained by them acting effectively in substancial dose in the cytoplamisc and mitochondrial superoxide dismutase respectively, maybe by the action of trace element.
Yet, there is a question: what logic reasoning or theoretical pattern must be elaborated to explain the lipidic peroxidation inhibition in the 30ª centesimal, that supplant the Avogrado number?
Stell observe that as the inhibition mentioned happened also with medicine whose dilution supplants the Avogrado number, we can infer the existance of antioxidant mechanisms stell inknown, that must be discovered.
Once verified the lipidic peroxidation inhibition, in rats brain homogenate in vitro, gotten by the use of homeopathic medicine, it’s inferred na antioxidant effect even bigger when used in vivo, considering the whole symptomatic of the individual.
This work considered only the lipidic peroxidation, however, it is open the possibility of studying the antioxidant action of the homeopathic medicine in the other phases of ROS cascades.
In general the LPx is the result of unbalance between pro and antioxidant favoring the first ones (Harata et al, 1983; Kawase et al, 1989).
The antioxidants are very important in the mechanism of action of several toxins. The oxidants involvement in several diseases is well established, however there isn’t unanimity about it ( Halliwell and Gutteridge, 1989).
Philogenetically and ontogenetically the organism develped antioxidant defense mechanisms, making possible the adaptation of superior organnisms to the aerobic environmente where the 3 enzymes superoxide dismutase, catalase and glutathione peroxidase are fundamental for the pro and antioxidant balance. The use of exogenous substances in order to mantain this balance can highly contribute to the human health.
The melatonin in substanteal dose showed it is a mighty antioxidant inhibiting satisfactorily the lipidic peroxidation. The idea of using melatonin in different concentrations was to give comparative support to the homeopathic medicine, because the melatonin has efficient antioxidant effect ( Halliwell and Gutteridge, 1989).
The confrontation of the homeophatic medicine highly diluted with substantial melatonin doses, as well as the description of their respective pathogeneses, serves as subsidy not only for a better understanding of their antioxidant effects but also to try to understand the own pathogeneses of these metals, extending this comprehension to the pathogeneses of the homeopathic medicine in general.
About the lipidic peroxidation inhibition gotten through homeopathic medicine experimentation we can infer that a thing happens because of the oligoelements action of such medicaments, or even through the proper homeopathic action described in the pathogeneses.
The reactive oxygen species are involved with an immense of physiological reactions, as well as in processes of diseases pretty known nowadays.
In front of such processes, the organism tries to adapt to the environment conditions where it lives through the homeostasys, denomination introduced to the Biology by the North American physiologist Walter Bradfort Cannom, in 1916. The homeostasys is the living being hereditary property of lasting long in time, maintouning the morphological and functional balance of its cells and tissues. The homeostasys is maintained by another hereditary property, the self-regulation (Maffei, 1978).
The homeostasys and self-regulation of the genotype constitute the organism adaptation and compensation mechanisms to the external agents, influencing this way not only at the time of a disease manifestation as well as in the way of evolution and therapeutic action (Maffei, 1978).
This adaptation happens mainly when we refer to the oxygen paradox. The living beings evolved passing from the anaerobic to the aerobic conditions, what happened thanks to the efficient antioxidant system development, where the enzymes superoxide dismutase, glutathione peroxidase and catalase play a fundamental role to the species preservation and perpetuation whose metabolism is aerobic (Halliwell and Gutteridge, 1985).
As the homeopathic medicine probably acts recovering the homeostasys, it is possible that it happens due to the antioxidant action of this medicament in rats brain homogenate.
Admitting the Homeopathy is based in the experimentation in vivo, it was assumed the possibility of demonstrating experimentally in vitro the antioxidant effect of the homeopathic medicine.
It is important to remember that long ago before Claude Bernard, HaHnemann used the experimentation in vivo, establishing, this way, the pathogenese (Tetau, 1980). On this perspective we can assure, by analogy, the functionality of the experimentation in vitro.
We start by the premise that the medicine is just one, only being variable the techniques and the therapeutic methods used by different schools.
Einstein said:
“Every religion, every art, every science are branches from the same tree where these aspirations Focus on the human life ennolument, raising it over the spheres of the existence merely material and leading the individual to freedom” ( Monteiro, 1982).
A controlled study was developed in a demonstrative way that aimed to demonstrate the of the experimental method in vitro.
It was based on the presupposition that, if the antioxidantion phenomenon happens isolated, in vitro, in the conditions verified by anology, there is a reasonable probability of being reproduced in na individual as a whole, once the mechanism for what its action happens is known and evaluated.
In order to delimit properly the study in such a universe, the conceitual and operational definition of the used terms was clarifild and it was also explained the focus over what the analysis was made (Andrade, 1995).
In this way, the study was concentrated on the lipidic peroxidation, suggesting however, the need of a better comprehension of the other oxidative chain phases, what would make possible a greater property in the homeopathic medicine application in the several diseases and organic changes.
The study stell aimed to measure the homeopathic medicine action in the immediate ambit of the experimental research, for a better understanding of its use in the human health.
This works opens perspectives for a better comprehension of the homeopathic medicine action as antioxidant, as well as its general action in the organism.
It important to point ou that, because fo the characteristics of the experiment it is impossible to elect the similimum medicament, what is supposed to have a more effective antioxidant action when administrated to the living being, filling the individual whole symptomatic.
6 CONCLUSIONS
The melatonin showed to be more efficient in the lipidic peroxidation in rats brain homogenate in 1 molar, concentration, coming next the melatonin 0,5 molar and the melatonin 0,25 molar.
The lipoperoxidation inhibition with the use of the homeopathic medicine occurred in a decreasing scale, like the following: cuprum metallicum C12, Cuprum metallicum C30, Arsenicum album C30, Manganum C30 and Arsenicum album C12.
The other groups analysed did not present significant differences.
It was observed significative inhibition of the lipoperoxidation with the use of homeopathic medicines over the Avogrado number, such as the cuprum metallicum C30 and the arsenicum album C30.
The homeopathic medicine produced significative inhibition of lipoperoxidation comparatively to the melatonin, showing na antioxidant action in vitro, deducing that, maybe, when administered according to the Law of similars positive answers can happen to the individual in this same sense.
7 ABREVIATIONS AND SYMBOLS
CN – Ion cyanide
CuZn SOD – Superoxide dismutase copper and zinc dependent
DNA – Desonyribonucleic acid
DOPA 3,4 – dihydroxyphenylalanine
DTPA – Diethylenetriamine-pentacetic acid
Ec SOD – Superoxide dismutase extracellular
ROS – Reactive Oxygen species
GPx – Glutathione peroxidase
GSH – Glutathione reduced
GSSG – Glutathione oxidated
Hb – Hemoglobin
HCN – Hydrogen cyanide; cyanidric acid
H2O2 – Hydrogen peroxide
HO∙ – Hydroxyl
KCN – Potassium cyanide
LPx – Lipoperoxidation
MDA – hyde
Mn SOD – Superoxide dismutase manganese dependent
NADP – Nicotinamide Adenine dinucleotide phosphate, reduced form
NADPH – Nicotinamide adenine dinucleotide phosphate, oxidate form
NBT – Blue of nitrotetrazole
O2 – Dioxygen; molecular oxygen
O2∙ – Superoxide
1O2 – Oxigênio Singlet
RNAm – Ribonucleic acid messager
RO∙ – Alcoxil radical
ROO∙ – Peroxil radical
ROOH – Organic Hydroperoxide
SOD – Superoxide dismutase
TBA – Tiobarbituric acid
TCA – Trichloroacetic acid
REFERENCES
AMES, B.N. – Dietary carcinogens and anticarcinogens – oxyten radicals and degenerative diseases, Science, 221:1256, 1983
AMES, B.N.& GOLD, L.S – Endogenous mutagens and the causes of aging and cancer: Mutat. Res., 250:3-16, 1991.
ANDRADE, M.M. – Como Preparar Trabalho para Cursos de Pós – Graduação. São Paulo. Ed. Atlas AS, 1995, 118 p.
BAROLLO, CR. Aos que se tratam pela Homeopatia. 4ª edição. São Paulo. Editora Typus, 1989, 93 p.
BAST, A; HAENEN, G.R.M.M.; DOELMAN, C.J.A – Oxidants and antioxidants: state of the art. Am.J.Med., 91 ( suppl 3C): 2S-13S,1991.
BEUTLER, E; WEST, C:BEUTLER, B. – Eletrophoretic polymorphism of glutathione peroxidase. Hum. Gent, 38:163-169, 1974
BRUNINI, C. Aforismos de Hipócrates, São Paulo. Typus IBEHE. 1998, 173 p.
CASTRO, D. Homeopatia e Profilaxia. São Paulo, Editoração Mauro Familiar Editora. 1980, 145 p.
CHADA, S; WHITNEY, C; NEWBURGER, PE – Post-transcriptional regulation of glutathione peroxidase gene expression by selenium in the HL-60 human myeloid cell line. Blood, 74 (7): 2535-2541, 1989.
CHANCE, B- Effect of pH upon the reaction kinetics of the enzyme-substrate compounds of catalase. J. Biol. Chem., 194(2):471-481, 1952a.
________ Effect of pH upon the reaction kinetics of the enzyme-substrate copounds of catalase. J. Biol. Chem, 194 (2): 483-496, 1952b.
CHANCE, B. & OSHINO, N – Dinetics and mechanisms of catalase in peroxisomes of the mitochondrial fraction. Biochem J., 122:225-233, 1971.
CIRIOLO, M.R., FISKIN, K. DE MARTINO, A; CORASANITI, M.T.; NISTICO, G; RTILIO G. – Age-related changes in Cu, Zn, superoxide dismutase, Se-dependent and – independent glutathione peroxidase and catalase activities in specific areas of rat brain. Ageing Dev., 61:287-297, 1991.
COHEN, G. & HOCHSTEIN, P. – Glutathione peroxidase: the primary agent for the elimination of hydrogen peroxide in erythrocytes. Biochemistry, 2(6): 1420-1428, 1963.
CUTLER, R.G. – Evolution of human longevity and the genetic complexity governing again rate. Proc.Natl.Acad. Sci. USA, 72(11): 4664-4668, 1975.
________ Human longevity and againg: possible role of reactive oxygen species. Ann NY Acad. Sci., 621:1-28, 1991.
DEMARQUE, D – Técnicas Homeopáticas. Buenos Aires, Ediciones Marecel, 1981, 328 p.
DUPRAT, H . A teoria e a Técnica da Homeopatia, Rio de Janeiro Gráfica Olímpica Editora, 1982, 198 p.
EATON, J.W – Catalase and peroxidases and glutathione na hydrogen peroxide: mysteries of the bestiary. J. Lab. Clin. Med., 118:3-4, 1991
FORSTRON, J W; ZAKOWSKI, JJ; TAPEL, A L. – Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochemistry, 17(13): 2639-2644, 1978
FRIDOVICH, I – The biology of oxygen radicals. Science, 201:875880. 1978.
GAETANI, G.F.; GALIANO, S; CANEPA, L; FERRARIS, A M; KIRKMAN, H N – Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood, 73(1): 334-339, 1989.
GERCHMAN, R; GILBER, D L ; NYE, S W; DWYER, P ; FENN W O – Oxygen poisoning and X-irradiation: a mechanism in common. Science, 119:623-626, 1954
GONZALEZ, M J – Lipid peroxidation and tumor grown: na inverse relationship. Med Hypotheses, 38 (2): 106-110, 1992
GUYTON, A C – Tratado de Fisiologia Médica. 4ª edição. Rio de Janeiro. Ed Guanabara Hoogan S/A, 1973, 974 p.
HALLIWELL, B. – Oxidants and human disease: some new concepts. FASEB J, 1: 358-364, 1987.
_______ Oxidants and the central nervous system: some fundamental questions. Acta Neurol. Scand., 126:23-33, 1989b.
_______ How to characterize a biological antioxidant. Free rad. Res. Commun, 9 (1): 1-32, 1990.
_______ Reactive ozygen species in living systems: source, biochemistry, and role in human disease. Am. J. med, 91 (suppl 3C): 14S-22S, 1991.
HALLIWELL, B & GROTTLVELD, M. – The measurement of free radical reactions in humans, FEBS Lett, 213(1):9-14, 1987.
HALLIWELL B. & GUTTERIDGE, J M C – Free redicals in Biology and Medicine. Oxford: Clerendon Press, 1985, 543 p.
________ Free radicals in Biology and Medicine. Oxford: Clarendon Press, 1989, 543 p.
HALLIWELL , B; HOULT, R; BLAKE D R – Oxidants, inflammation, and anti-inflammatory drugs. FASEB J, 2:2867-2873, 1988.
HAHNEMANN, S. Matéria Médica Pura. 2ª edição. New Delhi. Jain Publishing Co, 1920.
_________ Doenças Crônicas – Sua natureza Peculiar e Sua Cura Homeopática. New Delhi. Jain Publishing Co, 1984
HARMAN, D – The aging process. Proc. Natl. Acad. Sci. USA, 78 (11): 7124-7128, 1981.
HENDLER, SS – Vitamin and Mineral Encyclopedia. San Diego, Simon e Schuski, 1990, 576 p.
KAY, M M B; BOSMAN, G J C G M; SHAPIRO, S S ; BENDICH, A ; BASSEL, P S – Oxidation as a possible mechanism of cellular aging: Vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc. Natl. Acad. Sci. USA 83: 2463-2467, 1986.
KENT, J T . Filosofia Homeopática, Buenos Aires. Editorial Albatroz. 1980, 342 p.
LATHOUD. Matéria Médica Homeopática. Buenos Aires. Editorial Albatroz, 1980, 868 p.
MACHLIN, L J & BENDICH, A – Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1:441-445, 1987.
MAFFEI, W E. – Os fundamentos da Medicina. 2º edição. São Paulo. Artes Médicas, 1978, 781 p.
MANNERVIK, B – Glutathione peroxidase. Methods Enzymol, 113:490-495, 1985.
MARKLUND , S L. – Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. USA, 79:7634-7638, 1982.
MASTERS, C & CRANE D – Isozymes and the micro organization of organellar structure and function. In: Isozymes: Structure, Function, and Use in Biology and Medicine. Wilwy-Liss, Inc, 1990. P 101-122.
McBRIDE, O W.; MITCHELL, A; LEE, B J; MULLENBACH, G; HATFIELD, D – Gene for selenium-dependent glutathione peroxidase maps to human chomosomes 3,21 and X BioFactors, 1 (4):285-292, 1988.
McCORD, J M & FRIDOVICH, I – Superoxide dismutase na enzymic function for erythrocuprein ( hemocuprein). J.Biol. Chem., 244 (22) 6049-6055, 1969.
MEERA KHAN, P; VERMA, C; WIJNEN, L M M.; JAIRAJ, S – Red cell glutathione peroxidase (GPX1) variation in Afro-Jamaican, Asiatic Indian na Dutch populations: is the GPX1*2 alled of “Thomas” variant na Afrincan marker? Hum. Genet., 66: 352-355, 1984.
MENETRIER, J . A medicina das funções. São Paulo. Organon, Bio. Press, 2000, 222 p.
MILLS, G C. – The purification and propertiers fo glutathione peroxidase of erythorcytes J Biol. Cheim., 234:502-506, 1959.
MITROPOULOS, D – Oligoterapia. Italy, Editorium Milano, 1994, 137 p.
MONTEIRO, I – Reflexões Filosóficas. Ed. São Paulo. Alvorada, 1982, 124 p.
PAGLIA, D E & VALENTINE, W.N. – Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med, 70(1): 158-169, 1967.
PERCY, M E. – Catalase: na old enzyme with a new roe? Can. J. Biochem. Cell Biol., 62:1006-1014, 1984.
PORTA, E. Tissue lipoperoxidation and lipofuscin accumulation as influenced by age, rype of dietary fat and levels of vitamin E in rats. Advances in the Biosciences, 64:37-74, 1987.
_______ Role of oxidative damage in the again process. In: CHOW, C.K., ed. Cellular Antioxidant Defense Mechanisms. Vol. III, Boca raton, FL: CRC Press, 1988. P. 1-52.
POZETTI, G.L. “Mecanismo de Ação de Medicamento Homeopático”. São Paulo, Revista de Homeopatia, 1986, 66 p.
PUSTIGLIONE, M & CARRILO JR., R. Organon da Arte de Curar de Samuel Hahnemann – Versão para o português sistematizada e comentada por Marcelo Pustiglione e Romeu Carrilo Jr. São Paulo, Homeopatia Hoje, 1994, 205 p.
REITER, J. R. & MAESTRONI, G J M. Melatonin in relation to the antioxidative defense and immune systems: possible implications for cell and organ transplantation, J. Mol. Med., 77:36-39,1999.
SCOTT, M.D.; LUBIN, B. H.; ZUO, L; KUYPERS, F A – Erythrocyte defense against hydrogen peroxide: preeminent importance of catalase. J. Lab. Clin. Med, 118:7-16, 1991.
SIE, H – Oxidative stress: from basic research to clinical application. Am. J.Med., 91 ( suppl 3C): 31S-38S, 1991.
SINET, P.M ; MICHELSON, A M ; BAZIN, A; LEJEUNE, J ; JÉROME, H – Increase in glutathione peroxidase activity in erythrocytes from trisomy 21 subjects. Biochem. Biophys Res. Commun, 67 (3): 910-915, 1975.
SINET, P M.; GARBER, P; JÉROME, H – Inativaction of human CuZn superoxide dismutase during expossure to superoxide radical and hydrogen peroxide Bull. Europ. Physiopath. Resp., 17 ( suppl.): 91-97, 1981
SLATER, T.F – Free radical mechanism in tissue injury. Biochem J, 222:1-15, 1984.
SMITH, L; THIER, S.O – Fisiologia. 2ª ed. São Paulo. Panamericana, 1990, 1260 p.
SUN, Y & OBERLEY, L. W. – The inibition of catalase by glutathione. Free Rad. Biol. Med, 7:595-602, 1989.
TETAU, M – Homeopatia. 6º edição. São Paulo, Andrei, 1980, 175 p.
TORTI, A . Gli oligoelementi nel futuro terapêutico. Milão. Giusepe Mario Ricchiuto Editore, 1988, 232 p.
VANNIER, L . Compendio de Matéria Médica Homeopática. Médico. Editorial Porrua. S. A 1979.
VIJNOVSKY, B. Sintomas – Clave de La Materia Medica Homeopatica – Buenos Aires – Editorial Albatros. 1978, 250 p.
WEACKER, P; MUELLER, C.R; MAYEROVA, A; GRZESCHIK, K L; ROPERS, H.H – Assignment of the gene coding for human catalase to the short arm of chromosome 11. Ann. Genét., 23(2): 73-77, 1980.
¹Facis – Health Science College Of São Pauo-Ibehe – Center Of Post-Graduation In Homeopathy
²Tutor
