REGISTRO DOI: 10.5281/zenodo.10048470
Tatiani Beida1
Maria Lígia Minucci2
Marcel Pereira Rangel3
ABSTRACT
Objective: Analyzing the side effects of antidepressants in children, awarding about the radical use, and exposing the bad sides of abusive uses. Methods: Integrative revision of bibliography literature about side effects of using antidepressants in children. For this, the research included studies with a maximum of 10 years from the date of publication. There were used date bases LILACS-BIREME (Latin-American Literature DataBase in Health Science), SciELO (Scientific Electronic Library Online), and PubMed (maintained by National Library of Medicine). The search is realized by the keywords based in the descriptors in Health Science (DeCS): antidepressants; adverse reaction; psychopharmacology; children and adolescents and their respective English terms. Results: the effect of psychotropic drugs is more active and straight in children. The use of antidepressants, for example, fluoxetine, was attributed to a bigger difficulty in the adaptation in adult life, as the changes in the behavior. Other reported effects include toxicity, weight gain, metabolic syndrome, developmental delay, and activation syndrome. Conclusion: The psychiatric disorders in childhood have peculiarities and they are different from how they are treated. It is essential to be aware of the damage caused by antidepressants in children and to know how to analyze the risks and benefits of using the medication.
Keywords: Adverse reactions. Psychotropic drugs. Children and adolescents.
INTRODUCTION
Antidepressants are usually used in the treatment of humor disorders such as depression, anxiety, and panic, regardless of age. According to Toledo¹ the psychiatric disorders, especially anxious syndrome, are present in about 9% of children, with estimated there is one in four and five children in the world with some psychiatric disorder, they show symptoms like anger, disquiet, and mainly late in the development.
Among the most prescribed antidepressants are the Selective Serotonin Reuptake Inhibitors (SSRI) and Tricyclics (ADT). SSRIs reversibly and selectively inhibit serotonin receptors, causing side effects to be milder compared to ADTs, as they only involve the serotonergic neurotransmission system. For this reason, the effects would be fatigue, dizziness, nausea, headache, and weight changes. Depending on the drug, there is also inhibition of the CYP2D6 enzyme, resulting in the appearance of a Serotoninergic Syndrome that usually occurs in association with MAOI, the symptoms would be lethargy, tremors, and change in body temperature. The therapeutic range of SSRIs is wide, with low lethality in overdoses and its efficacy is superior to ADT.²
ADTs block the recapture of monoamines such as norepinephrine (NE), serotonin (5-HT), dopamine (DA), and histamine. It has side effects such as constipation, dry mouth, cardiotoxicity, and antimuscarinic effects. Due to the greater variety of receptors for which ADTs have an affinity, there are more systemic side effects, as already mentioned, and it also makes the response to treatment in an individual way, as it alters the neurotransmission of various systems. Therefore, its side effects associated with its low efficacy in children mean that the drug is little used, giving space to the SSRI. In addition, children have a faster maturation of the serotonergic system than the noradrenergic and dopaminergic ones, making the SSRI more used compared to ADTs.3 According to Moreira4, other antidepressants such as bupropion and venlafaxine, which are second-line agents, are used when the SSRI is not effective. Monoamine oxidase inhibitors (MAOI) are rarely prescribed because they need a special diet and have serious adverse effects such as hypertensive crises and seizures.
The treatment is divided into three phases: initial therapeutic experiment, continuation, and maintenance phase, it is necessary to consider that all antidepressant treatment is prolonged, this obtains a therapeutic response after 4 weeks of use. This delay in obtaining a therapeutic effect occurs in the first phase because of mechanisms of sensitization and desensitization of receptors and a dose-response curve, which may even exacerbate some symptoms. For example, patients with panic disorder may develop nervousness or increased panic attacks after starting treatment with tricyclics or SSRIs.5
Adverse effects appear early and must be distinguished from the usual symptoms presented by the patient’s illness. Therefore, it is noted that the prescriber must observe some important factors: less serious side effects, evaluating the previous response of the patient and family members, in addition to the physician’s experience with the psychotropic drug. One factor to be analyzed is that children and adolescents often need a higher dose by weight than adults to achieve the therapeutic effect, this is because the metabolism is faster by the liver and has an increased glomerular filtration, which may make therapy difficult and increase risks for the child.4
Another reported effect of antidepressants is the activation syndrome, characterized by the presence of disinhibition, impulsivity, insomnia, restlessness, hyperactivity, and irritability. This phenomenon was identified in children and adolescents undergoing treatment with SSRIs and selective serotonin and norepinephrine inhibitors (SSRIs), being responsible for the increase in the suicide rate and the abandonment of treatment. According to Luft6, these symptoms are usually more severe in prepubescent and vary according to the drug’s plasma level, genetic and neurochemical factors.6
Thus, it is observed that medicine prescription is a complex process, which involves knowledge of both the medicine and the user’s implications. It is important that the doctor explains in detail how the medicine works, its correct dosage, and its possible side effects.7 The prescriber must effectively transmit to parents as much information as possible about the disease, this helps parents to be able to recognize us your children some warning signs and symptoms, suicidal thoughts, or marked anhedonia. This is essential to identify the effectiveness of the treatment and create a doctor-patient bond, which is important for the child’s safe recovery.3
Treatment with antidepressants in children has its particularities, considering that it is a phase marked by physical and psychological development, making this population very susceptible to Pande adverse effects.8 In the literature, adverse effects have appeared as Dress’s Syndrome, worsening of depression, and suicide attempts, in addition to neuroleptic syndromes and metabolic problems. A problem with these effects is that most studies on the efficacy and safety of antidepressants are carried out in adults, and these medications are used by children without tracking their effects in this specific audience.8
For this reason, the child’s follow-up must be multidisciplinary, including pharmaceutical evaluation. The awareness of the population about the benefits and harms of the medicine is essential to reduce cases of intoxication by antidepressants, as well as proper management by the professional, who needs to recognize the best form of treatment.9
Studies on the effects of antidepressants in children have advanced considerably in recent years, but a large part of the population does not have information about these medications and they are often used and prescribed incorrectly. For this reason, the work aims to help health professionals to monitor a patient using antidepressants, objectively offering the aspects studied so far on the subject and also the unknowns to be answered.
Considering the impact of psychiatric disorders in childhood is essential to intervene in the use of medications. Thus, knowledge about its side effects, specifically in this target audience, is essential to provide correct treatment and quality of life, as well as recognizing not only the biological impacts on the child but also the psychological ones on the patient and family.
METHODS
This literature review included randomized clinical trials and reviews published in English and Portuguese. The databases LILACS-BIREME (Database of Latin American Literature in Health Science), SciELO (Scientific Electronic Library Online), and PubMed (maintained by the National Library of Medicine) were used. The search period was between January 2020 and September 2021.
The search was performed using the Health Sciences Descriptors (DeCS) in the Portuguese language: Antidepressant, adverse reactions, psychopharmacology, children and adolescents; and their respective terms in English: Antidepressant, adverse reactions, psychopharmacology, children and adolescents. For the selection of articles, the title and abstract of the articles were carefully read, and if considered relevant to the study, a complete interpretive reading of the study was carried out. Subsequently, in vitro and animal research studies, as well as case studies and case reports, were excluded.
RESULTS
In the systematic search, 473 articles were found, 464 of which were excluded for being results that did not fit the selection criteria. By manual search, 7 articles were found and included, resulting in 480 studies, of which 464 were disregarded by the exclusion criteria. At the end of the process, 16 articles remained. The “IACAPAP Child and Adolescent Mental Health Treaty” by Boris Lorberg, published in 2020, was also included in the results. The flowchart was used to represent the results, as shown in figure 1.
Figure 1. Flowchart of the result of articles found for the search.
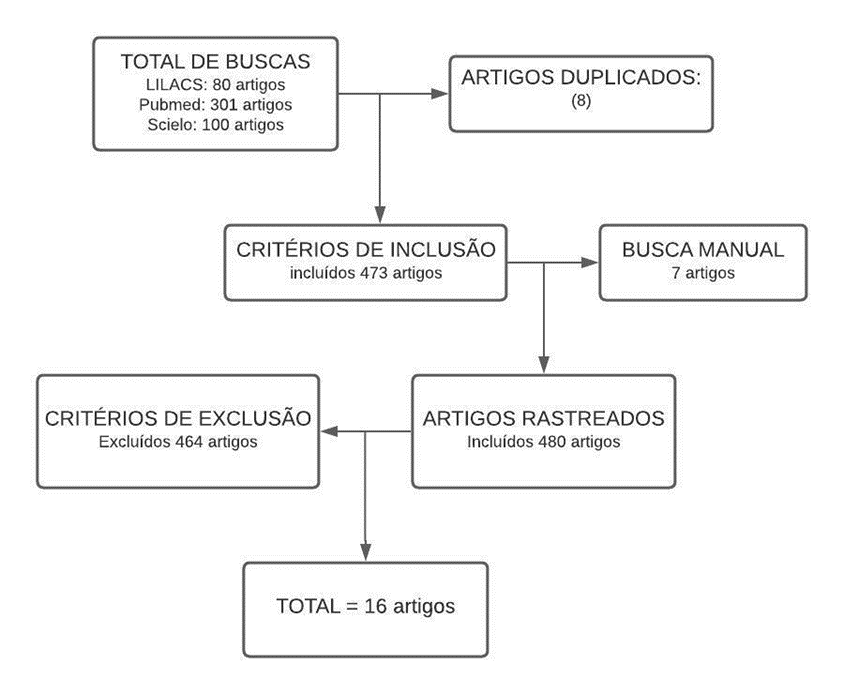
The articles that contain results related to the adverse effects of the use of antidepressants in children are described in table 1.
Table 1 : Results of article analysis
DISCUSSION
After analyzing the articles, there is a significant increase in the number of children and adolescents diagnosed with mental syndromes that require pharmacological treatment. According to Thiengo10, 16.5% of adolescents suffer some psychiatric disorder and, in Brazil, 7 to 12.7% of these are underdiagnosed situations. Among the most common psychiatric pathologies, ADHD is shown with 8.3%, where the figures in Brazil show that 17% of public school students, aged between 6 and 15 years, are diagnosed with this disorder and need to use antidepressants. In some situations, treatment or self-medication is abandoned, which ends up generating adverse effects.10 In addition to ADHD, Major Depressive Disorder (MDD) is also very present in this age group, and the risk of developing this disorder increases as childhood progresses, 50% of patients with MDD are said to experience their first episode before age 20.3
Treatment with psychotropic drugs in children and adolescents is essential to reduce the damage caused by mental disorders. However, its indication should start from a risk-benefit assessment and not as an immediate solution, trivializing the possible risks that may occur.4 Still analyzing the risks-benefits that medication with antidepressants can bring, we have an example in 2004, when the US Food and Drug Administration (FDA) has issued a warning about the use of antidepressants in children. The basis for this was that these medications caused suicidal thoughts and behavior. Thus, in 2007 data were presented that showed a decrease in the use of SSRIs from the note published by the FDA. However, concomitant with this decrease, there was a significant increase in suicide rates (an increase of 49% between the years 2003 and 2005), which shows that the benefit of using SSRIs outweighed the risk.12
The use of medicines such as antidepressants, anxiolytics, and antipsychotics, even though they are considered safe, have undesirable effects, among the most common are: toxicity, dystonias, appetite suppression, dyskinesia, metabolic syndrome, suicidality, growth delay, weight gain, irritability, insomnia, disinhibition, impulsivity, restlessness, and hyperactivity. For this reason, the safety of prescribing these drugs in children and adolescents is controversial in the literature.
The study by Pande8 concludes that the use of psychotropic drugs is associated with a longer patient stay in the health system compared to treatment with psychotherapy alone. The author also reports that 50% of medications do not show satisfactory evidence in childhood and adolescence, and their use does not take into account the physiological particularities of this age group.
However, the work by Cousins12 states that 60% of depressed patients aged 10 to 17 years experienced remission of symptoms with the use of fluoxetine and that the use of SSRIs is a protective factor against suicide. This is supported by the 49% increase in suicides in the US in this age group between 2003 and 2005, a period which, as recommended by the Food and Drug Administration (FDA), was not prescribed SSRIs for the treatment of psychiatric disorders for patients underage. 20 years.
The study by Topczewski13 also shows positive results in the use of antidepressants in adolescents with ADHD. A tricyclic antidepressant and antipsychotic compound, specifically imipramine (1.2 mg/kg/day) and chlordiazepoxide (0.05-0.08 mg/kg/day), was used to treat 140 adolescents with ADHD. The results showed improvement in 71.43% of patients, mainly in sleep quality and school performance. Only 20% had side effects in the initial phase of treatment, such as reduced appetite, dry mouth, constipation, headache, and palpitations. For this reason, the study justifies the use of the medication, considering that the benefits outweighed the risks caused by the treatment.
In recent years, more studies have been done to discover adverse effects in children and adolescents. In addition to these studies, the reports of parents and guardians of children who use medications were also important. One of the studies was carried out with newborn mice. Fluoxetine was manipulated by them and analyzed for the effects of this medicine. The result was transient inhibition of the serotonin transporter. This was related to reduced exploratory behaviors, such as slower adaptation to new environments or decreased stimuli in adulthood. That is why it is so important to be cautious when treating children who are still developing, especially those under 6 years of age.14
According to Muniz9, a study carried out in 2016 in Pouso Alegre – MG analyzed the records of 74 patients diagnosed with antidepressant intoxication. The result was that 57 are female and the most prevalent age group with drug poisoning was 21 to 30 years. Between the age group 0 to 20 years, there were a total of 13 intoxicated patients (17.56%), thus showing that children and adolescents represent a low number with intoxication by antidepressants. The same study reported that the most used drug in intoxication is the benzodiazepine class, including Clonazepam, Diazepam, and Rivotril.
Some adverse effects appear after hours or days of using the drug, such as dystonias caused by dopaminergic antagonists or appetite suppression by stimulants, others are evident later, they can take months to years, such as dyskinesia or metabolic syndrome with antipsychotics. Dyskinesia can also appear through a rebound effect after antipsychotic withdrawal.14
Costa15 carried out a study with 30 people, aged 7 to 17 years, intending to compare the efficacy of fluoxetine and clomipramine with placebo in the treatment of anxiety disorder. Within the study, some side effects of the medications were identified. Fluoxetine presented sedation, malaise, abdominal discomfort, excessive salivation, tachycardia, and excessive sweating as adverse reactions. While clomipramine had a feeling of confusion more often than fluoxetine. However, even though fluoxetine had the most adverse effects, it was the drug with the highest rate of symptom improvement. The study result was unexpected, as the placebo-treated group also had a high response. This can be explained by the fact that, during the study, the young people who participated had educational monitoring about the disorder and quality of doctor-patient contact, in addition to the very expectation that the clinical trial would bring improvements.15
Some effects are better diagnosed by clinical evaluation, in physical exams, for example. Stimulant medications such as methylphenidate and amphetamines can interfere with children’s physical growth (height and weight). After 14 months of stimulant treatment for ADHD, children grew an average of 1.4 cm shorter than children treated with behavioral therapy. Furthermore, it was analyzed that this effect was maintained in the following years in children who continued treatment with stimulants for 3 years. Besides, it was more evident in children who were at the beginning of puberty. As for weight gain, second-generation antipsychotics were more likely to gain in children than in adults.14
Another side effect restricted to the studied group is the activation syndrome, which includes symptoms such as disinhibition, impulsivity, insomnia, restlessness, hyperactivity, and irritability. Activation is related to the plasma level of the drug and the patient’s age, with younger age and a diagnosis of bipolar disorder being important risk factors. In the work by Luft6, a survey of patients using fluoxetine who presented activation syndrome was reported, 5 out of 7 of them did not continue treatment, with the syndrome being the main reason for discontinuing treatment.
The response to SSRIs is associated with biochemical and genetic factors, especially the activation syndrome, and early effects occur mainly through genetics. A study with candidates aged 7-18 years using citalopram evaluated genetic variants among them and concluded that alterations in the 5-HTR1Db gene (CC Genotype) are related to agitation and risk factors for suicide, being more present in males. Thus, it is important to consider this information when indicating drug treatment, to minimize the risks of iatrogenesis. However, there are not many studies that explore genetics in response to psychotropic drugs.16
Rates of child suicide attempts have increased considerably in recent years. Studies reveal that most of these patients seek medical care or professional help with somatic complaints one month before a suicide attempt, so it is important to identify the risk and initiate treatment. The first weeks of antidepressant treatment are associated with an increase in the suicide rate, precisely due to the side effects, for this reason, consent must always be obtained, not only from the guardians but also from the treated child.17
In the study by Strawn18, escitalopram and placebo were used to treat pediatric anxiety disorders. A patient who used a placebo had hospitalization due to verbal aggression and increased irritability as an adverse effect; one patient using escitalopram had an aborted suicide attempt. On the Columbia Suicide Severity Rating Scale (C-SSRS), during the study, there were 6 worsening events in subjects using escitalopram, while on placebo there were 2 events. Even with these data, the final result did not show a significant difference in the worsening and/or the emergence of suicide in young people treated with escitalopram and placebo.18
When analyzing that both children and adolescents are in a very important neurodevelopment stage and are influenced by the environment, the use of antidepressants can improve or worsen the relationship with the environment and directly affect the individual’s development. Even so, failing to treat depression ends up having bad long-term results.12
Therefore, adolescents and children should not be considered versions of depressed adults, as they react differently to a treatment. Another differential is related to the tolerability of antidepressants, and an effect described in children related to this factor is activation syndrome. The activation event is characterized as a set of signs and symptoms, such as restlessness, disinhibition, impulsivity, insomnia, hyperactivity, and irritation. Therefore, activation is related to a decrease in the drug’s efficacy and an increase in discontinuation. This phenomenon involves genetic and neurochemical factors, appearing at the beginning of treatment or when the dose is increased. For this reason, the physician must be aware of the risk factors and changes in the patient’s behavior, for example, female, prepubescent, with generalized anxiety are great indicators of activation syndrome, is recommended to start with low doses of antidepressants and non-pharmacological measures.6
CONCLUSION
There is a great increase in psychiatric disorders in childhood and studies on the particularities in their treatment are still inconclusive. Numerous psychological and biological factors differentiate the use of antidepressants in children, such as the fact that they are still in the process of neurodevelopment, and for this reason, the therapeutic approach in pediatrics must be different from the traditional one, associating several areas of health. In most studies, SSRI treatment was considered safe, but all antidepressant treatments should be risk-benefit-weighted to have as few side effects as possible. However, the harm of going without the drug is still greater than the side effect, in the short term. Studies are needed to find out if this also prevails in the long term.
INDIVIDUAL CONTRIBUTIONS
All authors contributed to the research and choice of articles, in the analysis and interpretation of data, in the elaboration of the article, as well as its review and final approval.
CONFLICT OF INTERESTS
Nothing to declare.
ACKNOWLEDGMENT
There was no source of funding.
REFERÊNCIAS
- Toledo, RRM. “Redução do uso de antidepressivos e benzodiazepínicos na comunidade Vila Santo Antônio no município de Imbituba-SC.”
- Von Werne Baes, C, Juruena, MF. “Psicofarmacoterapia para o clínico geral.” Medicina (Ribeirão Preto, Online.) 50.Supl 1 (2017): 22-36.
- Pereira, JGPM. “Depressão na Infância e Adolescência: Revisão da Literatura.” (2016).
- Moreira MS, Morais RG, Moreira EA, Leite SF, Teixeira CC, Silva ME, et al. “Uso de psicofármacos em crianças e adolescentes.” Revista da Universidade Vale do Rio Verde 12.2 (2014): 1013-1049.
- Sadock BJ, Sadock VA, Ruiz P. Compêndio de Psiquiatria-: Ciência do Comportamento e Psiquiatria Clínica. Artmed Editora, 2016.
- Luft MJ, Lamy M, DelBello MP, McNamara RK, Strawn JR. “Antidepressant-induced activation in children and adolescents: risk, recognition and management.” Current problems in pediatric and adolescent health care 48.2 (2018): 50-62.
- Marques, ALN, Ferreira MBG, Duarte JMG, Costa NS, Haas, VJ, Simões ALA. “Qualidade de vida e contexto de trabalho de profissionais de enfermagem da Estratégia Saúde da Família.” Rev Rene 16.5 (2015): 672-681.
- Pande MNR, Amarante PDC, Baptista TWF. “Este ilustre desconhecido: considerações sobre a prescrição de psicofármacos na primeira infância.” Ciência & Saúde Coletiva 25 (2020): 2305-2314.
- Muniz JJ, Batista LCB, Souza VPR. “Avaliação do índice de intoxicação por antidepressivos nos pacientes atendidos no pronto socorro do Hospital das Clínicas Samuel Libânio de Pouso Alegre-MG.” Revista de Ciências da Saúde Básica e Aplicada 1 (2019): 48-57.
- Thiengo, DL, Cavalcante, MT, Lovisi, GM. “Prevalência de transtornos mentais entre crianças e adolescentes e fatores associados: uma revisão sistemática.” Jornal Brasileiro de Psiquiatria 63 (2014): 360-372.
- Valença RCP, Guimarães SB, Da Paixão Siqueira L. “Prescrição e uso de antidepressivos em crianças e adolescentes–uma revisão da literatura.” Brazilian Journal of Development 6.12 (2020): 94860-94875.
- Cousins L, Goodyer IM. “Antidepressants and the adolescent brain.” Journal of Psychopharmacology, 29.5 (2015): 545-555.
- Topczewski, Abram. “Attention deficit and hyperactivity disorder: a therapeutic option.” Einstein (São Paulo) 12 (2014): 310-313.
- Lorberg B, Davico C, Mart Senkovski D, Vitiello B. “PRINCÍPIOS DO USO DE MEDICAÇÕES PSICOTRÓPICAS EM CRIANÇAS E ADOLESCENTES.” (2020).
- 15. Costa CZG, Morais RMCB, Zanetta DMT, Turkiewicz G, Neto FL, Morikawa M, Rodrigues CL, Labbadia EM, Asbahr FR. Comparação entre clomipramina e fluoxetina para o tratamento de transtornos de ansiedade em crianças e adolescentes (2013).
- Amitai M, Kronenberg S, Carmel M, Michalovsky E, Frisch A, Brent D, et al. “Farmacogenética dos efeitos colaterais relacionados ao citalopram em crianças com depressão e / ou transtornos de ansiedade.” Journal of neural transmission 123.11 (2016): 1347-1354.
- Toniazzo PB, Gomes CG, Rocha GP. “Risco de suicídio infantil: quando os sonhos quase terminam.” Acta méd.(Porto Alegre) (2014): 6-6.
- Strawn JR, Mills JA, Schroeder H, Mossman SA, Varney ST, Ramsey LB, Poweleit EA, Desta Z, Cecil K, DelBello MP. Escitalopram in Adolescents With Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J Clin Psychiatry. 2020.
1Graduating in Medicine- Centro Universitário Cesumar-UNICESUMAR
Maringá-PR
2Graduating in Medicine- Centro Universitário Cesumar-UNICESUMAR
Maringá-PR
3Professor Doutor – Centro Universitário Cesumar – UNICESUMAR
Av. Guedner, 1610 Jardim Aclimação
CEP 87050-390 – Maringá-PR
Telephone number:(44) 3027-6360 Ramal 1284
marcelprangel@gmail.com
