REGISTRO DOI: 10.5281/zenodo.7689373
Carlos Alberto Alves Dias-Filho1,7,8
Joseana Araújo Bezerra Brasil Pinheiro6
Andressa Coelho Ferreira1
Nivaldo de Jesus Soares Jr1
Alice S. Ferreira5
Alexsandro Guimarães Reis7
Wermerson Assunção Barroso7
Mariana Barreto Serra7
Monique Nayara Coelho Muniz Cardoso7
Sally Cristina Moutinho Monteiro3,5
Silvana Maria Moura da Silva2
Francisco Navarro2
Carlos José Dias1,2
Cristiano Teixeira Mostarda1,2,4
Background: Spinal cord injury (LM) above T6 is followed by a loss of supraspinal sympathetic control of the heart, disrupting autonomic balance and increasing cardiovascular risk. Objective: To evaluate cardiac autonomic modulation, biochemical markers, and sleep quality in wheelchair users with spinal cord injury and strength training. Methods: A 12-week cross-sectional study included 50 male participants aged between 20 and 52 years. With the evaluation of anthropometric measurements and body composition, Biochemical evaluation, Measurement of blood pressure, Pittsburgh Sleep Quality Index (PSQI), Injury Level, Assessment of the heart rate variability. Results: they point to a better HRV behavior in the wheelchair basketball (WCB) and wheelchair basketball + strength training (WCBS) groups to wheelchair control (WC). These data indicate that basketball practice and strength exercise combined with basketball practice can positively affect autonomic modulation. Conclusion: The practice of basketball, associated with resistance training, may improve hemodynamic, metabolic, and autonomic parameters.
Keywords: Cardiac autonomic modulation; Strength training; Basketball; Modular Injury
INTRODUCTION
Spinal cord injury usually occurs in young adults and is very disabling because it results in motor, sensory and autonomic deficits 1. It’s essential to mention that sympathetic innervation to the heart is disturbed in lesions above T6 because of damage in the afferent and efferent pathways to sympathetic neurons located in the T1-T5 medullary segments. The parasympathetic innervation of the vagus nerve is also unchanged, causing an autonomic imbalance 2,3. Consequently, after spinal cord injury (SCI), the disorder in autonomic balance leads to increased cardiovascular risk 4.
A sedentary lifestyle is typical after SCI, contributing to cardiovascular disorders responsible for the increased risk of cardiovascular diseases in wheelchair users 5. Studies showed that aerobic and resistance exercise has pointed to an essential non-pharmacological intervention to reduce several risk factors related to cardiovascular diseases in SCI, such as glucose impairment 6,7, atherogenic lipid profile 8, uric acid 9 sleep quality, and reduced cardiovascular fitness 10.
Additionally, it is well documented that cardiac autonomic function can improve after regular exercise in healthy individuals with spinal cord injury 11,12. However, there is still a lack of studies in the literature that demonstrate how often the combination of sports practice could lead to positive cardiovascular changes and whether the combination with resistance training could potentiate or impact parameters such as hemodynamic variables, metabolic, autonomic, and sleep quality 13 14.
The practice of sports in the rehabilitation process increases physical activity and improves the quality of life, leading to self-esteem recovery15. However, despite cardiac autonomic impairment, biochemical changes, and poor sleep quality in individuals with SCI, few studies have reported the importance of basketball sports practice, combined with strength training in cardiac, biochemical, and sleep quality autonomic control in people with SCI.
Thus, this study evaluated cardiac autonomic modulation, biochemical markers, and sleep quality in wheelchair users with spinal cord injury and strength training.
METHODS
A 12-week cross-sectional study included 50 male participants aged between 20 and 52 years. The study divided participants into four groups: Healthy Sedentary, Control (C) (11 individuals); Control wheelchair users (WC) (15 individuals); basketball wheelchair users (WCB) (13 individuals), and wheelchair basketball ball plus strength training 12 weeks (WCBS) (11 individuals). All methods used in this study are approved by the Institutional Ethics Council (Opinion Number: 2,639,370), (CAAE: 87424618.5.0000.5087) and follow the guidelines Helsinki declaration. Before starting the research, all methods were presented to the participants and entered the study voluntarily after signing the free and informed consent form.
As a criterion of non-inclusion, the participants of the wheelchair groups could not: 1- current diagnosis with multiple spinal lesions; 2- being smokers; 3- Be wheelchair users without spinal cord injury or 4- make use of cardiovascular medications. The wheelchair users participated in the study after a public call. The physical education department of the Federal University of Maranhão was the site of data collection. All data collection was performed on days and times previously scheduled or according to the availability of volunteer athletes. The subjects avoided caffeine, alcohol, and physical activity 24 hours before the test.
The composition of the WC group occurred through wheelchair users of an association of people who have spinal cord injury and are sedentary; we structured the WCB and WCBS group by basketball players in wheelchairs of the Parasport Excellence Center team. The group that trained basketball had a regular workout, twice a week between 2 and 3 hours a day.
Regarding strength training, the agonist/antagonist method was followed, consisting of performing two exercises respecting the sequence, first the training of the agonist’s muscle, then its respective antagonist applicable to all muscle groups 16. We performed the strength exercises sitting in his wheelchair of daily locomotion or sitting in the gym equipment. The sequence of the exercises performed was: 1. Front handle (back); 2. Sitting straight supine (chest); 3. Direct thread (biceps); 4. French triceps (triceps); 5- Lateral elevation (shoulder, medial deltoid); 6- frontal elevation (shoulder, anterior deltoid); 7- Thread handle (arm); 8- Inverted handle thread (forearm). Strength training was performed in twelve consecutive weeks, three times a week for an hour on average.
EVALUATION OF ANTHROPOMETRIC MEASUREMENTS AND BODY COMPOSITION
The size of the participants’ height, the subject lay on the stretcher in the supine position and with anthropometric metric tape (Sanny TR4010) with an accuracy of 0.1 cm 17.
We checked the body mass with a digital scale (Welmy) with a precision of 0.05 kg 18, which enlarged the base area of the scale to support the bodyweight of wheelchair users, invited them to sit in a sturdy lightweight plastic chair with the support arm. Weighed the chair and base for the scale previously and deducted from their total body mass value.
We measured waist circumference at the supine position and the umbilical scar point. We used a flexible anthropometric metric Trena (Sanny TR4010), with an accuracy of 0.1cm for this measurement. We measured at the end of expiration without compression of the measuring tape19.
We measured the right and left arms circumferences with the individual sitting in the wheelchair or not, with the arms lost next to the body and the hands facing the thigh. And we made all measurements with the same flexible, inelastic tape (Levolpe)20.
To determine the body mass index of the participants in this study, we used the method recommended by the World Health Organization and those recommended by Rajan et al. (2008) and Laughton et al. (2009), is estimated, dividing the mass of an individual (in kg) by his height (in m 2) 21,22
BIOCHEMICAL EVALUATION
We used sterile and disposable materials to perform venous blood samples (8 ml) fasting from 10 to 12 hours. The samples were properly packed and transported to the analysis site 23, the clinical biochemistry laboratory of the Pharmacy Department of UFMA. Where all the recommendations of the priest touch interteck semi-automatic biochemistry analyzer and the kits (Bioclin) used to evaluate, Fasting glycemia (mg/dl) were followed; Uric acid (mg/dl), total cholesterol (CT) (mg/dl); triglycerides (TG) (mg/dl); LDL cholesterol (mg/dl) and HDL cholesterol (mg/dl).
MEASUREMENT OF BLOOD PRESSURE
Two automated blood pressure monitors were used (Omron® HEM-711 and Omron® 905) for blood pressure measurement. The protocol followed the norms of the Brazilian Hypertension Guideline 24. An optimal cuff size was used according to the arm size of the participants 24.
PITTSBURGH SLEEP QUALITY INDEX (PSQI)
Sleep quality and sleep disorders were evaluated using the Pittsburgh Sleep Quality Index (PSQI) as initially defined by Buysse 15,25. The PSQI uses seven components: (a) subjective quality of sleep, (b) sleep latency, (c) duration of sleep, (d) habitual sleep efficiency, (e) sleep disorders, (f) use of medication to sleep, and (g) daytime sleepiness and disorders during the day. The score for each component was determined separately, on a scale of 0 to 21 points, where the higher the value of the score obtained, the worse is the quality of sleep. Score values between 0 and 4 represent good sleep quality, those between 5 and 10 represent poor sleep quality, and those greater than 11 indicate sleep disorders. PSQI score cut-point of ≥ 5 was used to differentiate high and low-risk groups for future cardiovascular events.
INJURY LEVEL
We used the American Spinal Injury Association (ASIA) impairment scale to assess the levels of injury and disability of wheelchair users 26. We classified all participants as A on the ASIA scale.
ASSESSMENT OF THE HEART RATE VARIABILITY
The heart rate signal was obtained using a 12-lead electrocardiogram device (Micromed Wincardio 1000 Hz, Brasilia, DF, Brazil) and recorded using WinCardio 6.1.1. For this evaluation, the students were instructed to remain at rest in the supine position for 10 min. We follow the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology for the analysis of HRV (40). The study of the HRV in the time domain expresses the results in unit time (milliseconds), measuring each average RR interval (sinus beats) during a specific time interval. Thus, based on statistical methods, the translator indices were calculated of fluctuations in the duration of cardiac cycles.
The indices were evaluated using the Kubios Analysis software HRV version 2.0 (Kubios, Finland). The ectopic beats (deviation higher than 20% of the adjacent intervals) were identified and manually interpolated by the adjacent R-R intervals. In the frequency domain, Fast Fourier Transform modeling was derived from all the data in a minimum 5-min window from the recorded signal. The total signal variance includes whether its frequency components appear as specific spectral peaks or nonpeak broadband powers. The frequency bands used low-frequency (LF, 0.04–0.15 Hz), high-frequency (HF, 0.15– 0.4 Hz), and autonomic balance (LF/HF), a component proposed as a measure of cardiac sympathovagal 27.
STATISTICAL ANALYSIS
The Chi-square test was used to analyze the association of injury level and group states. It was performed using GraphPad Prism 5.0 (La Jolla, CA, USA). The Kolmogorov- Smirnov test was used to verify the normality of distribution. The data were also treated by descriptive statistics. For comparisons between groups, the one-way ANOVA test with Tukey’s post hoc test for independent samples, or its nonparametric equivalent, Mann-Whitney U-test. Results were considered statistically significant at an alpha level of P<0.05.
RESULTS
Table 1 shows the level of injury and the ASIA classification of wheelchair users. No significant changes were found between the groups, using the chi-square test.
Table 1 : Injury Level and ASIA Classification in WC, WCB, and WCBS Groups
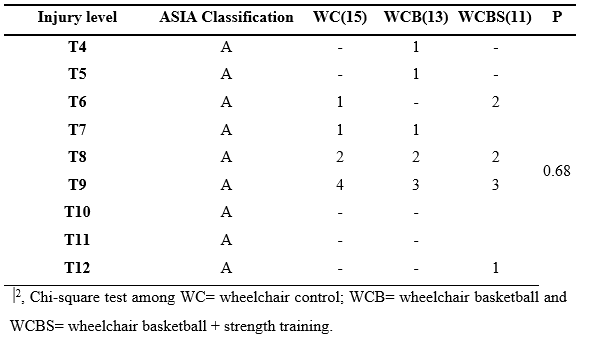
Table 2 : Sample characterization
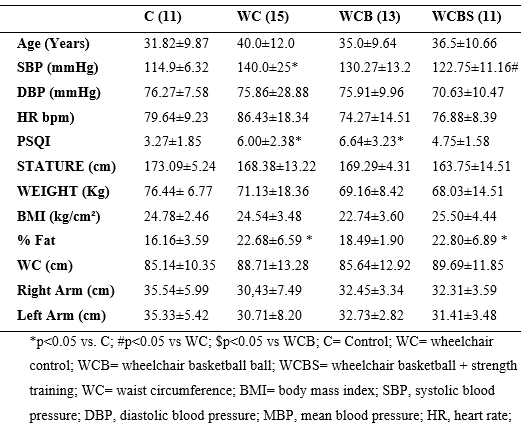
Among the characteristic data (table 2), it was possible to identify changes when analyzed group C with the WC and WCBS groups. They are indicating an increase in the %fat variable in these two last groups. The sleep variable was increased in two groups when compared to group C, as well as, SBP was also increased in the WC group when compared to group C.
Still, on the SBP variable, it was possible to identify a decrease in the WCBS compared to the WC group. In addition to the WCBS group, it presented a better quality of sleep when compared to the WC group. The other variables did not show statistically significant changes.
Figure 1 : The research groups indicate blood glucose, triglycerides, uric acid, cholesterol, LDL, and HDL
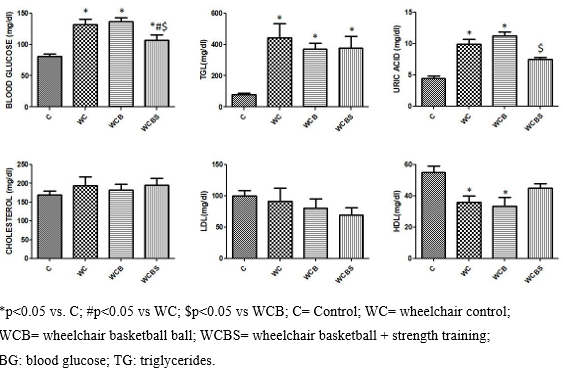
Figure 1 shows that the WC and WCB have higher blood glucose levels, TGL, uric acid, and lower HDL than C. However, the WCBS has lower blood glucose levels, higher HDL, and lower uric acid concentrations compared to the WC. Additionally, the WCBS group also presents lower blood glucose and uric acid compared to the WCB group. The variables cholesterol and LDL did not show significant alterations in any of the groups. Group C shows an expected behavior of a healthy individual in each of the variables.
Figure 2 : Comparison of the Values Obtained in heart rate variability evaluated in the domain of time and frequency between Groups C= Control; WC= wheelchair control; WCB= wheelchair basketball ball and WCBS= wheelchair basketball ball + strength training
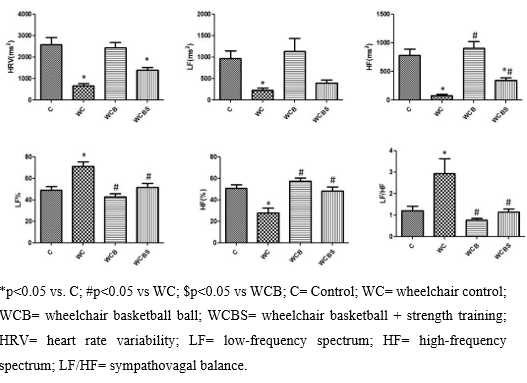
In Figure 1. The WC group presents a worse picture related to autonomic variables when compared to the other groups, which was evidenced by the increase in LF(%), LF/HF, and a fall in HF (%). However, the WCB group presented higher HF (ms²), HF (%), lower LF (%), LF/HF when compared to the WC group and did not present significant changes in the variables LF (ms²) and HRV (ms²). The WCBS group has lower LF, higher HF (ms²), HF (%), and HRV when compared to toilets. In addition to a better LF/HF.
DISCUSSION
This study evaluated the adaptations promoted by resistance training in wheelchair users with spinal cord injury, basketball practitioners in autonomic modulation, and metabolic parameters. Several pieces of evidence in the literature demonstrate that wheelchair users with spinal cord injury present an imbalance in the autonomic balance 4,28. These data indicate that basketball practice and strength exercise combined with basketball practice can positively affect autonomic modulation. On the other hand, our results suggest a better HRV behavior both in the WCB group and in the WCBS group to the WC. Fact not yet, not shown in the literature to the point where it was researched.
The improvement of HRV in the WCB, when compared to a WC, corroborates what was observed in the study Gomes et al. 20185, which identified improvements in HRV, HF, LF/HF, and lower LF components of autonomic modulation in basketball-practicing wheelchair users; these indicators represent the overall spectrum behavior 29. Aerobic exercise can lead to this type of response, caused by improved cardiopulmonary capacity due to moderate or high intensity, which leads to changes in autonomic modulation shown by the research participants 5,30,31. A fact is also observed in healthy individuals who demonstrate improved autonomic function after regular exercise 11. In addition, respiratory frequency and volume is the primary mechanism that influences the modulation of sinoatrial node activity, which has repercussions on rapid fluctuations in the HF range of HRV 32.
Additionally, strength training seems to affect the health of patients with spinal cord injury positively. Studies conducted with wheelchair athletes 1,33 have reported benefits of physical exercise, such as increased muscle strength, exercise performance of the upper limbs, and improved quality of life 34. The literature demonstrates that an acute resistance training session can significantly impact cardiac autonomic modulation 35.
Resistance training probably positively affects HRV in individuals with cardiac autonomic dysfunction. Chen et al. (2011) report a significant decrease in HF potency and a significant increase in relative LF potency after a resistance exercise session using four different exercises in healthy young men. This result makes these improvements evidenced in the present study because the literature has already demonstrated that patients with spinal cord injury have reduced heart rate variability 2,36. This autonomic imbalance leads to an increase in cardiovascular risk 4. Although the effects of resistance training on HRV in healthy individuals have been well documented, the impact of resistance training on modulation autonomy in special populations is less known.
Still, in this context, it is commonly accepted that a lower HRV is associated with a higher cardiovascular risk and mortality in healthy individuals 37. And cardiovascular diseases represent one of the leading causes of morbidity and mortality after spinal cord injury 28. They indicate the importance of improvements in risk indicators for wheelchair users.
In the present study, it was possible to observe that systolic blood pressure (SBP) in the WCBS group presented better values when compared to the WC group, thus decreasing one of the cardiovascular risk factors for wheelchair users. It is well known that regular exercise at moderate and high intensity can lead to decreased resting BP38,39. They corroborated the study by Haddad, silva, Barreto, and Ferraretto 1997 and Castro et al. 202040,41, which showed a decrease in SBP in wheelchair users practicing physical activities upper-limbs training programs and basketball practice, respectively. In other studies, it was possible to indicate that regular physical exercise may decrease resting BP in people with paraplegia (LM at T1 or below) 42,43.
Furthermore, improvements in sleep quality in the WCBS group were evident. This improvement found in the present study indicates a decrease in cardiovascular risks for the WCBS group since it is demonstrated in the literature that poor sleep quality contributes to cardiac autonomic dysfunction in this population, with an increase in cases of nocturnal dyspnea 44.
The intensity of physical exercise may also interfere with sleep quality. The study by Albu et al. 2019, by regression analysis, reported that more intense physical activity help improves sleep quality 45, which contributes to reducing such cardiovascular risks. In the WC and WCB groups, it was possible to identify that they had worse sleep quality than the other groups, which may be justified by the lack of training in the WC group or by a lower training intensity in the WCB group.
The present study also presented a higher percentage of fat in the WC and WCBS group participants than group C, corroborating the study by Eskici and Ersoy 2016 and Quintana and Neiva 200846,47. The increase in adipose tissue has been associated with impaired metabolism of carbohydrates and lipids in people with spinal cord injury (SCI) 48. Another important fact is that disabling conditions such as overweight and obesity are identified 49.
According to the study by Andrew et al. 2013 50, it was possible to identify the adiposity index, lean body mass, physical activity with heart rate variability, and cardiac vagal control markers. They are associated as indicators that show that increased physical activity, low visceral fat concentration, and higher lean mass indicate more significant heart rate variability and, therefore, better cardiac function and cardiac performance and lower cardiovascular risk.
In addition, it is essential to draw attention to the lipid glucose and profile of participants where it was possible to observe lower blood glucose values and higher HDL values only in the WCBS group. Regarding glycemia, the WCBS group presented a lower value when compared to the WC and WCB groups. Some studies show decreased blood glucose in disabled people who practice adapted sports and reduced CVD risk 51,52. These corroborating other studies observed a positive relationship of improvement in lipidogram and physical activity in wheelchair users 40,47,53,54. This improvement could be associated with higher expression of GLUt4 and improved insulin resistance 55.
Another marker that was possible to find decreased values in the WCBS was uric acid (AU), which is closely correlated with almost all known cardiovascular risk factors 56, insulin resistance 57, metabolic syndrome 58, obesity 59, non-alcoholic fatty liver disease 60 and chronic kidney disease 61. In many cases, there is a mutual relationship between the AU and these conditions. In this sense, a high uric acid level can be seen as a marker of cardiovascular risk because uric acid is a product of xanthine oxidase (XO), responsible for most reactive oxygen species production (ROS) in the body. The elevated level of AU may be a marker or consequence of regulated or increased ERO activity and increased oxidative stress. ERO is related to CVD 62.
The results of the present study are significant because they corroborate the findings of Trapé et al. 2013 63 and indicate the value of sports practice, especially those combined for the control of these measures, thus decreasing cardiovascular risks for both healthy people and in SCI fact not yet demonstrated in the literature to date. We believe that our results point to a favorable contribution of resistance training in metabolic, hemodynamic, and autonomic parameters in basketball players.
CONCLUSION
Basketball practice associated with resistance training may improve hemodynamic, metabolic, and autonomic cardiac parameters.
REFERENCES
1. Roberto Zamunér A, Silva E, Macher Teodori R, Maria Catai A, Aparecida Moreno MJJoss. Autonomic modulation of heart rate in paraplegic wheelchair basketball players: Linear and nonlinear analysis. 2013;31(4):396-404.
2. Buker DB, Oyarce CC, Plaza RSJTiscir. Effects of spinal cord injury in heart rate variability after acute and chronic exercise: a systematic review. 2018;24(2):167-176.
3. Krassioukov AJTiscir. International standards to document remaining autonomic function after spinal cord injury (ISAFSCI), 2012. 2012;18(3):282.
4. West C, Bellantoni A, Krassioukov AJTiscir. Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. 2013;19(4):267-278.
5. Gomes RL, Diniz RR, Silva-Filho AC, et al. Effect of Regular Wheelchair Basketball Practice on Autonomic Modulation and Sleep Quality of Spinal Cord Injured Subjects. 2018;21(3)
6. Graham K, Yarar-Fisher C, Li J, et al. Effects of high-intensity interval training versus moderate-intensity training on cardiometabolic health markers in individuals with spinal cord injury: a pilot study. 2019;25(3):248-259.
7. Vivodtzev I, Taylor JAJJoCR, Prevention. Cardiac, Autonomic, and Cardiometabolic Impact of Exercise Training in Spinal Cord Injury: A QUALITATIVE REVIEW. 2021;41(1):6-12.
8. Farrow M, Nightingale TE, Maher J, et al. Effect of exercise on cardiometabolic risk factors in adults with chronic spinal cord injury: a systematic review. 2020;101(12):2177-2205.
9. Ndrepepa GJCca. Uric acid and cardiovascular disease. 2018;484:150-163.
10. Farkas GJ, Gorgey AS, Dolbow DR, Berg AS, Gater Jr DRJTiSCIR. Energy expenditure, cardiorespiratory fitness, and body composition following arm cycling or functional electrical stimulation exercises in spinal cord injury: a 16-week randomized controlled trial. 2021;27(1):121-134.
11. Laing ST, Gluckman TJ, Weinberg KM, et al. Autonomic effects of exercise-basedcardiac rehabilitation. 2011;31(2):87.
12. de JS Soares-Junior N, Dias-Filho CA, Dias CJ, et al. Active Lifestyle can Contribute to Attenuation of Cardiac Autonomic Dysfunction in Adolescent Offspring of Hypertensive Parents. 2019;22(3)
13. Smith DJJSm. A framework for understanding the training process leading to elite performance. 2003;33(15):1103-1126.
14. Kuipers H, Keizer HJSM. Overtraining in elite athletes. 1988;6(2):79-92.
15. Bertolazi AN, Fagondes SC, Hoff LS, et al. Validation of the Brazilian Portuguese version of the Pittsburgh sleep quality index. 2011;12(1):70-75.
16. Uchida MC, Charro MA, Bacurau RFP. Manual de musculação: uma abordagem teórico-prática do treinamento de força. Phorte Editora LTDA; 2009.
17. Oliveira JDdF, Vargas LM, Oliveira Gd, Vargas TM, Gutierrez GL, Gorla JIJREFF. Métodos de medidas e avaliação antropométrica em indivíduos com lesão medular: uma revisão sistemática. 2015;6(2)
18. Gorla JI. Educação Física Adaptada: o passo a passo da avaliação. Phorte Editora LTDA; 2009.
19. Gorgey AS, Gater DRJAP, Nutrition,, Metabolism. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. 2011;36(1):107-114.
20. Rio de Janeiro R. Diretrizes do ACSM para os testes de esforço e sua prescrição.[Google Scholar]. 2013;
21. Rajan S, McNeely MJ, Warms C, Goldstein BJTjoscm. Clinical assessment and management of obesity in individuals with spinal cord injury: a review. 2008;31(4):361-372.
22. Laughton G, Buchholz A, Ginis KM, Goy RJSc. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. 2009;47(10):757-762.
23. Berte LMJCilM. Laboratory quality management: a roadmap. 2007;27(4):771-790.
24. Barroso WKS, Rodrigues CIS, Bortolotto LA, et al. Diretrizes Brasileiras de Hipertensão Arterial–2020. 2021;116:516-658.
25. Dias Filho CAA, Dias CJ, Barroso R, et al. Cardiac autonomic modulation of adolescents with different levels of sleep quality. 2020;13(4):224.
26. Jha AJEocn. ASIA Impairment Scale. 2011:255-257.
27. Electrophysiology TFotESoCtNASoPJC. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. 1996;93(5):1043-1065.
28. Garshick E, Kelley A, Cohen S, et al. A prospective assessment of mortality in chronic spinal cord injury. 2005;43(7):408-416.
29. Dias‐Filho CAA, Soares NdJS, Bomfim MRQ, et al. The effect of family history of hypertension and polymorphism of the ACE gene (rs1799752) on cardiac autonomic modulation in adolescents. 2021;48(2):177-185.
30. Pereira RN, Abreu MFR, Gonçalves CB, Corrêa WFS, Mizuhira DR, Moreno MAJMRdEF. Respiratory muscle strength and aerobic performance of wheelchair basketball players. 2016;22:124-132.
31. St Croix CM, Morgan BJ, Wetter TJ, Dempsey JAJTJop. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. 2000;529(2):493-504.
32. Malik MJAoNE. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. 1996;1(2):151-181.
33. Goosey-Tolfrey V, Castle P, Webborn NJBjosm. Aerobic capacity and peak power output of elite quadriplegic games players. 2006;40(8):684-687.
34. Hicks A, Martin K, Ditor D, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. 2003;41(1):34-43.
35. Heffernan KS, Sosnoff J, Jae S, Gates G, Fernhall BJIjosm. Acute resistance exercise reduces heart rate complexity and increases QTc interval. 2008;29(04):289-293.
36. Bunten DC, Warner AL, Brunnemann SR, Segal JLJCAR. Heart rate variability is altered following spinal cord injury. 1998;8(6):329-334.
37. Chen J-L, Yeh D-P, Lee J-P, et al. Parasympathetic nervous activity mirrors recovery status in weightlifting performance after training. 2011;25(6):1546-1552.
38. Cornelissen V, Verheyden B, Aubert A, Fagard RJJohh. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. 2010;24(3):175-182.
39. Stand APJMSSE. Exercise and hypertension. 2004;36:533-553.
40. de Castro KCE, Guimarães ACG, Silva GJ, et al. Estratificação do risco cardiovascular em cadeirantes jogadores de basquetebol. 2020;53(2)
41. Haddad S, Silva PRS, Pereira Barretto AC, Ferraretto IJABdC. Efeito do treinamento físico de membros superiores aeróbio de curta duração no deficiente físico com hipertensão leve. 1997;69:169-173.
42. Flank P, Fahlström M, Boström C, Lewis JE, Levi R, Wahman KJJorm. Self-reported physical activity and risk markers for cardiovascular disease after spinal cord injury. 2014;46(9):886-890.
43. Haddad S, Silva PRS, Pereira Barretto AC, Ferraretto IJAbdc. The effect of short term Aerobic physical training using upper limbs in paraplegic persons with mild to moderate hypertension. 1997;69(3):169-173.
44. Tobaldini E, Proserpio P, Sambusida K, et al. Preserved cardiac autonomic dynamics during sleep in subjects with spinal cord injuries. 2015;16(6):779-784.
45. Albu S, Umemura G, Forner-Cordero AJScs, cases. Actigraphy-based evaluation of sleep quality and physical activity in individuals with spinal cord injury. 2019;5(1):1-9.
46. Eskici G, Ersoy GJTJoSM, fitness p. An evaluation of wheelchair basketball players’ nutritional status and nutritional knowledge levels. 2014;56(3):259-268.
47. Quintana R, Neiva CMJRBdMdE. Fatores de risco para síndrome metabólica em cadeirantes: jogadores de basquetebol e não praticantes. 2008;14:188-191.
48. Gorgey AS, Mather KJ, Gater DRJM. Central adiposity associations to carbohydrate and lipid metabolism in individuals with complete motor spinal cord injury. 2011;60(6):843-851.
49. Platt LSJJoat. Medical and orthopaedic conditions in Special Olympics athletes. 2001;36(1):74.
50. Andrew ME, Shengqiao L, Wactawski‐Wende J, et al. Adiposity, muscle, and physical activity: predictors of perturbations in heart rate variability. 2013;25(3):370-377.
51. Nooijen CF, de Groot S, Postma K, et al. A more active lifestyle in persons with a recent spinal cord injury benefits physical fitness and health. 2012;50(4):320-323.
52. Manns PJ, McCubbin JA, Williams DPJAopm, rehabilitation. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. 2005;86(6):1176-1181.
53. Phillips SM, Stewart BG, Mahoney DJ, et al. Body-weight-support treadmill training improves blood glucose regulation in persons with incomplete spinal cord injury. 2004;97(2):716-724.
54. D Chilibeck P, A Guertin PJCpd. Locomotor training and factors associated with blood glucose regulation after spinal cord injury. 2017;23(12):1834-1844.
55. Fonseca GFAC. Efeitos de um protocolo de treinamento de subida em escada sobre variáveis relacionadas à resistência à insulina em camundongos alimentados com dieta hiperlipídica. 2020;
56. Strasak A, Ruttmann E, Brant L, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83 683 Austrian men. 2008;54(2):273-284.
57. Zhu Y, Hu Y, Huang T, et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. 2014;447(4):707-714.
58. Pacifico L, Cantisani V, Anania C, et al. Serum uric acid and its association with metabolic syndrome and carotid atherosclerosis in obese children. 2009;160(1):45.
59. Mangge H, Zelzer S, Puerstner P, et al. Uric acid best predicts metabolically unhealthy obesity with increased cardiovascular risk in youth and adults. 2013;21(1):E71-E77.
60. Petta S, Cammà C, Cabibi D, Di Marco V, Craxì AJAp, therapeutics. Hyperuricemia is associated with histological liver damage in patients with non‐alcoholic fatty liver disease. 2011;34(7):757-766.
61. Lee JE, Kim Y-G, Choi Y-H, Huh W, Kim DJ, Oh HYJH. Serum uric acid is associated with microalbuminuria in prehypertension. 2006;47(5):962-967.
62. Battelli MG, Bolognesi A, Polito LJBeBA-MBoD. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. 2014;1842(9):1502-1517.
63. Trapé AA, Jacomini AM, Muniz JJ, et al. The relationship between training status, blood pressure and uric acid in adults and elderly. 2013;13(1):1-7.
1Cardiovascular Exercise Adaptation Laboratory – LACORE, Federal University of Maranhão, Department of Physical Education, São Luís, Brazil;
2Professor – UFMA – Department of Physical Education;
3Professor-UFMA–Pharmacy Department.
4Cristiano.mostarda@gmail.com, Phone number: 3272-9270;
5Clinical Biochemistry Laboratory
6Link with the Maranhão State Education Department
7Faculdade de Medicina – ITPAC SANTA INÊS – AFYA.
8Faculdade Santa Luzia Santa Inês- MA.
