REGISTRO DOI: 10.69849/revistaft/th102412272000
Teixeira, Maria Clara Lopes 1
Jesus, Kézia França De 1
Araújo, Ludmila Carvalho De 2
Soares, Isabella Melo 2
Nascimento, Ginivaldo Victor Ribeiro Do 2, 3, 4
Abstract
Background: Acute kidney injury (AKI) is associated with high mortality rates, particularly among older adult (OA) in intensive care units (ICUs). Serum creatinine (SCr) variation serves as a key indicator for the timely detection of AKI. In OA, apparently normal SCr levels and inaccurate baseline estimated glomerular filtration rate (eGFR) may hinder prompt and effective interventions. This study aimed to analyze discrepancies in baseline eGFR using the Berlin Initiative Study (BIS)-creatinine and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations in OAs developing AKI after ICU admission. Additionally, it assessed eGFR levels at AKI diagnosis and their correlation with mortality. Methods: From January 2020 to March 2021, a retrospective study was conducted at a tertiary hospital in Brazil. Patients aged ≥ 60 years admitted without AKI were followed for up to 30 days or until ICU transfer, discharge, or death. Results: Out of a total of 203 older adults, only 36 developed AKI after admission to the ICU, with the majority being excluded due to pre-existing AKI prior to hospitalization to the ICU; the majority were female (66.7%) with an average age of 71.67 years. All patients required mechanical ventilation, and 91.7% received vasopressors. Only 25% of the patients were notified by nephrologists. Despite initial SCr appeared normal (0.87 ± 0.22 mg/dL), eGFR values indicated reduced renal function, particularly using BIS-creatinine (33% vs 18.2%, CKD-EPI). According to KDIGO classification, 69% were stage 1. Mortality was 88.9%, associated factors were age (odds ratio [OR] 1.2, 95% confidence interval [CI]: 1.07-1.5, p = 0.049), persistent AKI (OR 16.20, 95% CI: 2.38-48.88, p = 0.026 and BIS-creatinine value < 60 mL/min/1.73 m² (OR 1.19, 95% CI: 1.07-4.79, p = 0.048). Conclusion: In this cohort, BIS-creatinine demonstrated at AKI diagnosis a superior performance in identifying older adults with reduced renal function compared to CKD-EPI. Lower eGFR values, especially with BIS-creatinine, were associated with increased mortality. Monitoring renal function through eGFR calculation, particularly BIS-creatinine, may serve as a crucial warning for the management of AKI in older adult ICU patients.
Keywords
Acute kidney injury, Older adult patients, Berlin Initiative Study (BIS) – creatinine, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), critically ill, intensive care unit.
BACKGROUND
Acute kidney injury (AKI) is associated with a high mortality rate, especially in older adult patients admitted to the intensive care unit (ICU) 1,2. Even a small acute reduction in kidney function can worsen a patient’s prognosis. Therefore, early detection and treatment of AKI can prevent its major complications 1,2,3.
The aging process can cause structural modification in the kidney, thereby influencing its function. Renal structural modification can lead to a reduction in cortical volume, progressive nephrosclerosis, and a decrease in the number of functional glomeruli, leading to decreased renal function 2,4-6.
The glomerular filtration rate (GFR) decreases with age at a rate of 6.3 mL/min/1.73 m² per decade and is associated with age-related loss of muscle mass 4. In contrast, serum creatinine levels can remain masked for a long time. Furthermore, older adults are at a high risk of acute kidney injury 4-6.
Therefore, monitoring renal function in this population, especially through creatinine-based equations for assessing baseline estimated GFR (eGFR), allows for a more accurate assessment than that of serum creatinine levels in isolation. The Berlin Initiative Study (BIS)-creatinine, a tool designed for older adults, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) are highlighted in this study.7,8
Thus, the aim of this study was to compare the performance of two creatinine-based equations for assessing baseline eGFR in older adult patients admitted to a tertiary ICU who developed AKI after admission. The selected tools for comparison were BIS-creatinine and CKD-EPI. Additionally, we sought to assess their association with mortality. Furthermore, we aimed to identify epidemiological factors and correlate them with the diagnosis of AKI according to the criteria set by Kidney Disease: Improving Global Outcomes (KDIGO).
METHODS
This was an observational, retrospective, longitudinal, descriptive, and analytical study conducted at the tertiary referral hospital for urgent and emergency care in the city of Teresina, the main town of a low- to middle-income state in Brazil (21st place of 27 states in Brazil regarding national gross domestic product) 9. All patients aged ≥ 60 years admitted to the general and neurotraumatology ICUs between 1 January 2020 and 31 March 2021 who developed AKI following ICU admission were evaluated. The patients were followed-up for a maximum of 30 days following ICU admission or until they were transferred out of the ICU, discharged from the hospital, or died.
This study was reviewed and approved by the local Committee of Research Ethics. Patients who were diagnosed with AKI prior to ICU admission or had medical records indicating advanced stage 5 chronic kidney disease (CKD) with baseline serum creatinine (SCr) ≥ 5 mg/dL or eGFR < 15 mL/min/1.73 m², patients on chronic dialysis treatment; age < 18 years old; kidney transplantation; patients without known previous SCr, whose SCr did not normalize (≤ 1.5 mg/dL), or whose SCr did not decrease by at least 50% from its peak value during hospitalization were excluded from this study. Additionally, patients whose medical records lacked renal function marker tests, laboratory tests, or ultrasound imaging (indicating renal atrophy) were excluded. Patients with missing or incomplete data in their medical records due to damage or errors in clinical and laboratory records that compromised the interpretation and analysis of essential data for this study were also excluded. 9-11
AKI was defined as an increase of > 0.3 mg/dL from baseline SCr within 48 hours or an increase in SCr to 1.5 times baseline, which is known or presumed to have occurred within 7 days, according to the KDIGO criteria. Patients with an SCr of 1.5 mg/dL or more, without known baseline SCr values and without SCr decrease, were viewed as having AKI only if history, renal ultrasound, and laboratory examinations were indicative of this diagnosis 10. In this case, we used an estimated baseline SCr or the lowest SCr value during their stay in the hospital, whichever was lower. The baseline SCr was estimated using the simplified Modification of Diet in Renal Disease (MDRD) formula, assuming a GFR of 75 mL/min per 1.73 m2 1,12. Another relevant data point concerns the eGFR, which was calculated based on the BIS-creatinine and CKD-EPI equations.7,8,13,15,16 For the BIS-creatinine equation, the calculation was performed using the following formula: BIS-creatinine “BIS-1” = 3736 × creatinine -0.87 × age -0.95; for female participants, the eGFR was multiplied by 0.82, whereas for the CKD-EPI equation, the calculation was performed using the following formula: eGFR = 141 × min (SCr/k, 1) α × max (SCr/k, 1) – 1.209 × 0.993 age × 1.018; for female participants, the eGFR was multiplied by 1.159.7,8,13,15,16 In this equation, SCr represents serum creatinine (mg/dL); “k” is 0.7 and 0.9 for women and men, respectively; “α” is −0.329 and −0.411 for women and men, respectively; “min” indicates the minimum SCR/k or 1; and “max” indicates the maximum SCR/k or 1. 7,8, 13-15
The severity of AKI was assessed based on the KDIGO staging criteria, where stage 1 is defined as an increase of ≥ 1.5 to 1.9 times above the baseline creatinine, stage 2 as an increase of ≥ 2 to 2.9 times above the baseline creatinine, and stage 3 as an increase of ≥ 3 times above the baseline creatinine or the initiation of renal replacement therapy. 1,17
The data were collected via forms specifically designed for this study. Only two researchers who were not involved in patient care collected data. The first form collected epidemiological information such as sex, age, race, and lifestyle habits, as well as comorbidities associated with the patient’s hospitalization. The second form collected information on the patient’s clinical progression, completed based on the medical record entries. These details were recorded according to the study’s inclusion and exclusion criteria. Thus, the form addressed whether the patient met the criteria for AKI according to KDIGO, clinical and laboratory progression, use of vasopressors, mechanical ventilation, and patient outcome.
Statistical analysis: All tests were two-sided, with a p-value of < 0.05 considered statistically significant. The data were analyzed using Statistical Package for the Social Sciences version 20.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as the mean ± SD or median with 25th and 75th interquartile ranges (IQR) based on the normality of their distribution assessed using the Kolmogorov-Smirnov test. Categorical variables were expressed as proportions and compared using Pearson’s chi-squared test. Multivariable logistic regression models were conducted using backward variable selection, with a P-value < 0.05 used for variable retention. Candidate variables included those with a likelihood ratio of significance < 0.2 in bivariate analysis. 9, 18-21. Variables were checked for multicollinearity by calculating the variance inflation factor. However, multicollinearity was not detected.
RESULTS
Out of a total of 203 older adults, only 36 were eligible they for analysis; the main reason for exclusion was that patients were admitted to the ICU already in the development of AKI. Twenty-four individuals (66.7%) were women with a mean age of 71.67 ± 8.72 years (mean ± standard deviation). Among the included patients, 31 (86.1%) came from the emergency department, 4 (11.1%) from the general ward, and 1 (2.8%) from other ICUs. Regarding notifications to the nephrologist, 27 (75%) patients did not notify after developing AKI. In general, 33 patients (91.7%) required the administration of vasopressors during hospitalization. All patients required mechanical ventilation support at some point during hospitalization, as shown in Table 1.
Table 1 – Baseline clinical characteristics of older adult patients who developed acute kidney injury during their intensive care unit admission (N = 36)
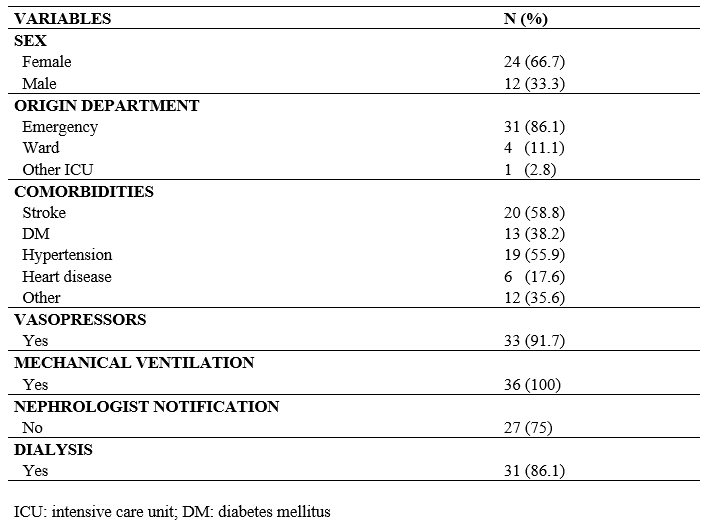
The most frequent comorbidities presented by the patients were stroke (58.8%), hypertension (55.9%), diabetes mellitus (38.2%), cardiomyopathy (17.6%), diverticular disease (8.8%), and other unspecified comorbidities (26.5%), resulting in an average of 2.04 (± 0.51) comorbidities per patient.
It was evident that 33% of the patients had BIS-creatinine values < 60 mL/min/1.73 m² upon ICU admission. Regarding CKD-EPI values upon ICU admission, 18.2% of the patients had values < 60 mL/min/1.73 m². Moreover, the BIS-creatinine at the time of AKI diagnosis was < 60 mL/min/1.73 m² for 86.1% of patients. Additionally, the CKD-EPI values at the time of AKI diagnosis were < 60 mL/min/1.73 m² for 84.6% of patients, as shown in Table 2.
Table 2 – Baseline clinical and laboratory characteristics of older adult patients who developed acute kidney injury during their intensive care unit admission (N = 36).
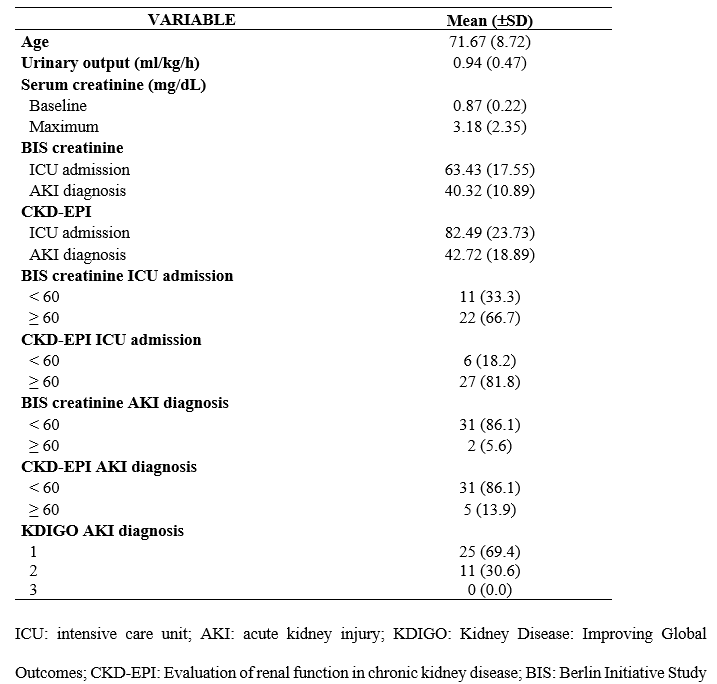
The occurrence of AKI detected at the time of diagnosis was classified according to the KDIGO criteria at the initial and peak injury moments. In the initial KDIGO classification, 69% of the patients were classified as stage 1 and 30.6% as stage 2 (Table 1). Furthermore, in the peak KDIGO classification, 36.1%, 22.2%, and 41.7% of the patients were classified as stage 1, stage 2, and stage 3, respectively.
According to laboratory parameters, the initial average and maximum creatinine levels of the patients were 0.87 ± 0.22 mg/dL and 3.18 ± 2.32 mg/dL, respectively, during hospitalization. Initial average and maximum average blood urea nitrogen levels were 28.85 ± 14.62 mg/dL and 80.49 ± 42.29 mg/dL, respectively.
Among the included patients, 32 (88.9%) patients died, and patient age was not identified as a risk factor for increased mortality in bivariate analysis. Similarly, no differences were observed in terms of sex, number of comorbidities, fluid balance, and urine output between the patients who died and survivors (Table 3).
Table 3 – Factors associated with increased mortality in older adult patients admitted to intensive care units who developed acute kidney injury. (N=36)
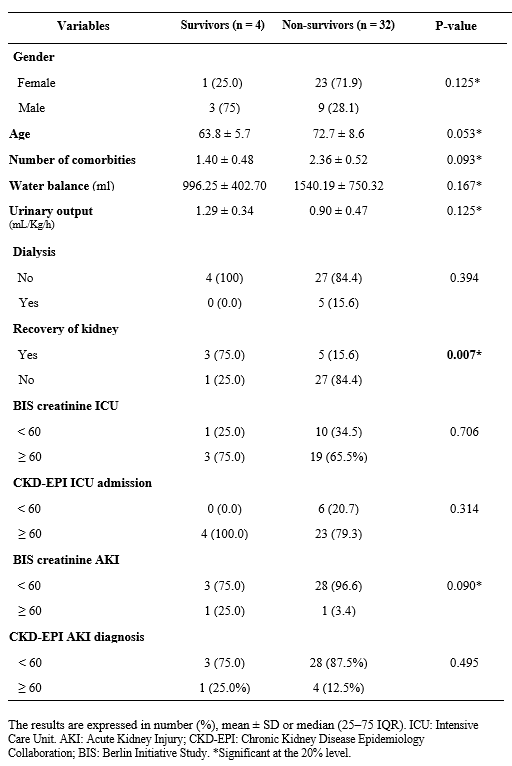
The results are expressed in number (%), mean ± SD or median (25–75 IQR). ICU: Intensive Care Unit. AKI: Acute Kidney Injury; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; BIS: Berlin Initiative Study. *Significant at the 20% level.
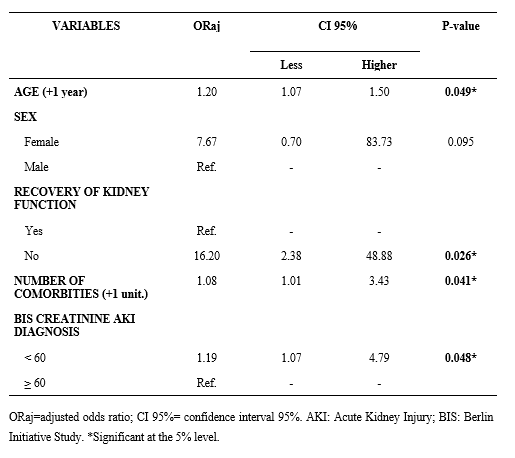
In the multivariate analysis, the factors associated with an increased likelihood of death were age (+1 year) (odds ratio [OR] 1.2, 95% confidence interval [CI]: 1.07-1.5, p = 0.049), persistent AKI (OR 16.20, 95% CI: 2.38-48.88, p = 0.026), number of comorbidities (OR 1.08, 95% CI: 1.0-3.43, p = 0.041), and BIS-creatinine value < 60 mL/min/1.73 m² (OR 1.19, 95% CI: 1.07-4.79, p = 0.048).
Table 4 – Multivariate analysis of death-related variables in older adult patients admitted to intensive care units who developed acute kidney injury.
DISCUSSION
The present study included 203 older adult patients. Among them, 36 patients were selected for the analysis based on the specified inclusion and exclusion criteria. These selected patients had a mean age of 71.67 years and a predominance of female patients in the study sample.
According to the Brazilian Society of Nephrology (SBN)22, the normal serum creatinine level range is broad, between 0.6 and 1.3 mg/dL and between 0.8 and 1.2 mg/dL according to Stevens et al 23. Additionally, serum creatinine values are influenced by variables such as age, sex, nutritional status, and muscle mass. Therefore, relying solely on plasma creatinine is not ideal for evaluating renal function, since elevated values above 1.3 mg/dL indicate a decrease of approximately 50–60% in GFR. In this regard, eGFR is the preferred measure, as it demonstrates an inverse relationship with creatinine 1,22-25.
Supporting this fact, older individuals, especially women, commonly experience lower muscle mass, rendering the evaluation of renal function through GFR more relevant.3,5,6,8 Relying solely on creatinine values may lead to a misclassification of these patients as falling within the “normal range”, even in the presence of decreased GFR. As observed in the present study, for patients diagnosed with AKI, the mean initial serum creatinine value of 0.87 mg/dL upon admission to the ICU was indicative of normal renal function6,8, 22, 23
However, this finding contrasts with eGFR values < 60 mL/min/1.73 m² upon ICU administration obtained using BIS-creatinine for 33.3% of the patients and using CKD-EPI for 18.2% of the patients. GFR values < 60 mL/min/1.73 m² indicated a reduction in the GFR and a manifestation of renal parenchymal injury.25 When this reduction persists for more than 3 months, it meets the diagnostic criteria for CKD according to KDIGO 25-28. Therefore, in this study, the values were categorized into GFR < 60 mL/min/1.73 m² and ≥ 60 mL/min/1.73 m².
As observed by Swedko et al. 29, serum creatinine is an unsatisfactory screening test for evaluating renal insufficiency in older adult patients, resulting in a significant underinvestigation and underrecognition of renal insufficiency in this population 3-7,16, 24. The results of this study were in accordance with these findings. According to Swedko et al. 29, older adult patients may have undetected CKD masked by “normal” serum creatinine values, which can be misclassified as having normal renal function 5,6,8, 29.
However, correct identification of this comorbidity is important for providing more comprehensive healthcare during follow-up and ICU admission, since patients with CKD who develop AKI may have worse outcomes than those without this comorbidity 3,6,29.
Nascimento et al. 9 reported that AKI patients aged > 60 years who were referred late to a nephrologist were associated with higher mortality. Furthermore, Nascimento et al. 9, in a retrospective observational study, observed that delayed consultation with a nephrologist is associated with an increased risk of death, even after adjustments are made (OR 2.66, 95% CI: 1.36-4.35, p = 0.001) 9.
This finding has been corroborated by Swedko et al. 29, who stated that a lack of proper investigation and referral of older adult patients with renal injury can increase the risk of morbidity and mortality; therefore, eGFR should be the preferred screening method for detecting renal injury in them 3,6,29.
Table 3 shows that patients who progressed to death often did not recover from AKI during a follow-up. AKI represents a significant complication in the ICU, particularly affecting older patients, with an overall incidence rate ranging from approximately 20-40% among admitted patients.30-32 According to Vijayan et al.30, recovery of kidney function tends to be less frequent in older patients, especially those with multiple comorbid conditions at baseline. In a study conducted by Hoste et al., it was observed that AKI occurs in approximately one to two-thirds of patients hospitalized in the ICU.32 Among these patients, approximately 10 to 15% require support with renal replacement therapy (RRT) 32. Despite therapeutic and diagnostic advancements, the mortality rate for this patient group has remained constant in recent years, at approximately 50% 3,5,6,30-32.
Additionally, according to Machado Levi et al.33, even small changes in serum creatinine levels can be associated with increased mortality. Consistent with this fact, the present study demonstrated that patients who progressed to death had an average maximum creatinine value approximately three times higher than the initial value, whereas these values were much lower for patients who did not experience death as an outcome (Table 3).
Certain comorbidities or conditions can impair or intensify renal function impairment, such as diabetes mellitus and systemic hypertension, thereby elevating the likelihood of developing kidney disease 6,7. In the present study, older patients with AKI presented similar characteristics and clinical conditions as patients in other studies in the literature, such as advanced age and various comorbidities, including stroke, diabetes mellitus, hypertension, cardiovascular disease, and diverticular disease. In the multivariate analysis, significant variables associated with mortality included age, comorbidities, and BIS-creatinine results (Table 4).
According to Teles et al. 34, older individuals are more prone to developing AKI and have higher mortality rates than the general population. Evidence suggests that dialysis in older adult patients does not yield positive survival outcomes, despite being a therapeutic method for life maintenance, especially in patients with existing comorbidities.3,6,34,35 According to the mentioned study, for each additional year of life, the risk of death increases by 20%. In other words, the older the patient is, the higher the risk of mortality.3,6,8,34,35
As presented in Table 4, for each additional comorbidity a patient experienced, the likelihood for mortality increased (1.08 times higher), with hypertension contributing significantly to the risk of death, as shown in Table 2.
The evaluation of eGFR methods, CKD-EPI and BIS-creatinine, demonstrated differing performance, with BIS-creatinine exhibiting a stronger correlation with mortality in older adult patients admitted to the ICU who subsequently developed AKI 7, 36. This finding aligns with that observed by Beridze et al.36 in a cohort of 3,000 older adult patients in Sweden, with a mean age of 78 years.
This study has certain limitations, as it is a retrospective observational study conducted at a single centre with a small sample size. Most of the patients were not admitted to the ICU in a timely manner, as indicated by the high percentage of patients requiring mechanical ventilation and vasopressor administration. This suggests that many patients may have already had AKI at the time of admission and were consequently excluded from this study. Despite these limitations, this study represented a real-world setting with ICU patients in a low-resource region of Brazil, where a small number were admitted without renal injury.
The results observed in the multivariate analysis coincided with findings from the existing literature and raise awareness about the inadequacy of serum creatinine as a renal function marker in older individuals, thereby suggesting the incorporation of BIS-creatinine as a useful tool for monitoring older adult patients admitted to ICUs.
CONCLUSION
In conclusion, in older adult patients aged > 60 years admitted to the ICU with stable renal function, the development of AKI during hospitalization, with an eGFR < 60 mL/min/1.73 m2 measured using BIS-creatinine, was associated with increased mortality (OR 1.19).
Conversely, this association was not observed when eGFR was measured using CKD-EPI. Additionally, increasing age and failure to recover renal function after an AKI episode were also associated with increased mortality, with ORs of 1.2 and 16.2, respectively. Due to the small sample size in this study, further research is necessary to confirm these findings. However, the present study encourages the increased use of eGFR measured by BIS-creatinine as a tool to help identify the levels of renal function that pose a risk for AKI and mortality.
Abreviations
AKI: Acute kidney injury
ICU: Intensive care unit
KDIGO: Kidney Disease: Improving Global Outcomes
GFR: Glomerular filtration rate
eGFR: Estimated glomerular filtration rate
ICU: Intensive care unit
BIS: Berlin Initiative Study
CI: Confidence interval
OR: Odds ratio
SCr: Serum creatinine
SD: Standard deviation
Declarations
- f1. Ethics approval and consent to participate
Local Committee of Research Ethics: Centro Universitário da Faculdade de Saúde, Ciências Humanas e Tecnológicas do Piauí – UNINOVAFAPI
Committee reference number: CAAE: 47727421.6.0000.5210
The need for informed consent was waived in this study, particularly in cases where logistical constraints presented challenges in locating or re-establishing contact with patients. The research is designed to ensure strict confidentiality, mitigating any potential impact on participants’ privacy
- f2. Consent for publication
Not applicable
- f3. Availability of data and material
The data that support the findings of this study are available from the corresponding author, GN, upon reasonable request.
- f4. Competing interests
The authors declare that they have no competing interests.
- f5. Funding
There are no grants or funding to disclose.
- f6. Authors’ contributions
GN conceived the study. MT, KJ, LA and IS substantially contributed to the acquisition of data, conducting the underlying review and drafting this manuscript. GN and IS produced the final version. All authors read, revised, and approved the final manuscript.
- f7. Acknowledgements
We thank professionals of the medical records section and professionals of the ICU from the Hospital de Urgências de Teresina who assisted in collecting data for the conduction of the study.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013; Suppl 3:1-150.
- Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021; 7:52.
- Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol.2011; 22:28–38.
- Denic A, Glassock RJ, Rule AD. Structural and Functional Changes With the Aging Kidney. Adv Chronic Kidney Dis.2016; 23:19-28.
- Chang-Panesso M. Acute kidney injury and aging. Pediatr Nephrol.2021; 36:2997-3006.
- Rosner MH, La Manna G, Ronco C. Acute Kidney Injury in the Geriatric Population. Contrib Nephrol.2018; 193:149-60.
- Corsonello A, Roller-Wirnsberger R, Di Rosa M, et al. Estimated glomerular filtration rate and functional status among older people: a systematic review. Eur J Intern Med.2018; 56:39–48.
- Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012; 157:471-81.
- Nascimento GVRD, Silva MN, Carvalho Neto JD, Feitosa Filho LR, Antão JD. Outcomes in acute kidney injury in noncritically ill patients lately referred to nephrologist in a developing country: a comparison of AKIN and KDIGO criteria. BMC Nephrol.2020; 21:94.
- Santos WJQ, Zanetta DMT, Pires AC, Lobo SMA, Lima EQ, Burdmann EA. Patients with ischaemic, mixed and nephrotoxic acute tubular necrosis in the intensive care unit – a homogeneous population? Crit Care. 2006; 10:R68.
- Yang, L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet.2015; 386:1465–1471.
- Luo X, Jiang L, Du B, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014; 18:R144.
- Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin Chem. 2007; 53:766-72.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604-12.
- Ebert N, Jakob O, Gaedeke J, et al. Prevalence of reduced kidney function and albuminuria in older adults: the Berlin Initiative Study. Nephrol Dial Transplant. 2017; 32:997-1005.
- Selistre LS, Rech DL, Souza V, Iwaz J, Lemoine S, Dubourg L. Diagnostic Performance of Creatinine-Based Equations for Estimating Glomerular Filtration Rate in Adults 65 Years and Older. JAMA Intern Med. 2019; 179:796-804.
- Ostermann M, Bellomo R, Burdmann EA, et al. Conference Participants. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020; 98:294-309.
- Nascimento GVRD, Balbi AL, Ponce D, Abrao JM. Early initiation of dialysis: mortality and renal function recovery in acute kidney injury patients. J. Bras. Nefrol. 2012; 34:337–42.
- Silva VT CE, Liaño F, Muriel A, Díez R, de Castro I, Yu L. Nephrology referral and outcomes in critically ill acute kidney injury patients. PLoS One. 2013;8: e70482.
- Mehta RL, McDonald B, Gabbai F, et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002; 113:456–61.
- Silva VT CE, Costalonga EC, APL O, Hung J, Caires RA, Hajjar LA, et al. Evaluation of intermittent hemodialysis in critically ill cancer patients with acute kidney injury using single-pass batch equipment. PLoS One. 2016; 11: e0149706.
- Sociedade Brasileira de Nefrologia (SBN). Biomarcadores na Nefrologia. Editor Hugo Abensur. Brasil: SBN. 2011; 1:1-114.
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function measured and estimated glomerular filtration rate. N Engl J Med. 2006; 354:2473-83.
- Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron. 2017; 136:302-08.
- Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023; 8:129.
- See EJ, Polkinghorne KR, Toussaint ND, Bailey M, Johnson DW, Bellomo R. Epidemiology and Outcomes of Acute Kidney Diseases: A Comparative Analysis. Am J Nephrol. 2021; 52:342-50.
- Charles C, Ferris AH. Chronic Kidney Disease. Prim Care. 2020; 47: 585-95.
- Ammirati AL. Chronic Kidney Disease. Rev Assoc Med Bras. 2020; 66: 3-9.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum Creatinine Is an Inadequate Screening Test for Renal Failure in Elderly Patients. Arch Intern Med. 2003; 163:356–60.
- Vijayan A, Abdel-Rahman EM, Liu KD et al. AKI!NOW Steering Committee. Recovery after Critical Illness and Acute Kidney Injury. Clin J Am Soc Nephrol. 2021; 16:1601-09.
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. The Lancet. 2019; 394: 1949-64.
- Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018; 14:607–25.
- Machado TL, Souza SP, Magalhães JG et al. Comparação dos critérios RIFLE, AKIN e KDIGO quanto à capacidade de predição de mortalidade em pacientes graves. Rev. Bras. Ter. Intensiva. 2020; 25:290-96.
- Teles F, Santos RO, Lima HMAM et al. The impact of dialysis on critically ill elderly patients with acute kidney injury: an analysis by propensity score matching. Braz. J. Nephrol. 2019; 41:14-21
- Li Y, Zhang D, Ma Q, Diao Z, Liu S, Shi X. The Impact of Frailty on Prognosis in Elderly Hemodialysis Patients: A Prospective Cohort Study. Clin Interv Aging. 2021; 16:1659-67.
- Beridze G, Vetrano DL, Marengoni A, et al. Concordance and Discrepancies Among 5 Creatinine-Based Equations for Assessing Estimated Glomerular Filtration Rate in Older Adults. JAMA Netw Open. 2023; 6: e234211.
1) UNINOVAFAPI- Coordenação Medicina
2) UNIFACID/Wyden – Coordenação Medicina
3) Universidade Estadual do Piauí – Centro de Ciências da Saúde, Coordenação Medicina
4) Hospital Universitário – Universidade Federal do Piauí – Setor da Gestão da Pesquisa e Inovação Tecnológica em Saúde
Corresponding author: Ginivaldo Victor Ribeiro do Nascimento
Campus I – Campus Universitário Ministro Petrônio Portela, SG 07 s/n – Ininga, Teresina – PI, CEP: 64049-550, Brazil
ginivaldovictor@gmail.com
