REGISTRO DOI: 10.69849/revistaft/ma10202509220819
Bárbara Loures Peralva1
Mário Flávio Cardoso de Lima2
Kelli Borges dos Santos3
Marina Guedes Fraga Lopes4
Cláudio Teodoro de Souza5
ABSTRACT
Aims: The present study aimed to synthesize the literature on the efficacy of treatments with a positive impact on reducing albuminuria in adult patients with metabolic syndrome (MetS). Methods: The present study followed the Joanna Briggs Institute (JBI) methodology for systematic reviews and meta-analyses. The inclusion criteria were studies evaluating treatments in adult patients with MetS. Studies describing treatments in children, pregnant women, animals and patients with type 1 diabetes were excluded. The databases selected for the search strategy were Cochrane, Embase, Scopus, Medline/PubMed and Dart-E Europe ETheses, without language restrictions. Studies since 2009 were included due to the consensus definition of MetS. Study selection and evaluation and data collection were performed by two independent reviewers. The search strategy was performed in August and September 2021. Results: Twenty-one articles were included, which showed that lifestyle changes and bariatric surgery seemed to reduce albuminuria in patients with MetS. Only three articles were included in the meta-analysis. In this analysis, diet alone or together with physical exercise was effective for reducing albuminuria in patients with MetS. Conclusions: Diet alone or associated with physical exercise has an impact on decreasing albuminuria in patients with MetS.
Keywords: Albuminuria; Proteinuria; Metabolic Syndrome; Drug Therapy; Healthy Lifestyle
1. Introduction
Metabolic syndrome (MetS) is characterized by a set of metabolic risk factors and is associated with an increased risk of cardiovascular disease and mortality 1. The incidence and prevalence of MetS have increased in recent years in both developed and developing countries, and MetS is considered a global public health problem 2. The components of MetS, particularly obesity and insulin resistance, are accompanied by changes in physiological functions in various systems and organs, including the kidneys.
A meta-analysis evaluated 10,603,067 individuals from 57 studies and found that some components of MetS, such as obesity, increased fasting glucose, systemic arterial hypertension (SAH) and hypertriglyceridemia, were associated with significant increases in the risks of proteinuria and albuminuria 3. In addition, the impact of MetS on proteinuria was more notable than that of each MetS component separately, highlighting the association between a greater number of MetS components and a higher risk of developing albuminuria 3. On the other hand, lifestyle changes, including changes in eating habits and physical exercise, have been proposed together with pharmacological therapy to control the components of MetS in important clinical studies 4.
Lifestyle modifications focusing on weight reduction and increased physical activity are the primary therapies for MetS control 5. Several dietary approaches have been studied for the treatment of MetS. Recent evidence has demonstrated a strong association between the incidence and prevention of MetS and modifiable lifestyle factors, especially dietary habits such as the Mediterranean diet, the Dietary Approach to Stop Hypertension (DASH), the ketogenic diet and a low-fat diet 6-9.
Regular physical activity has been recommended for the prevention of and rehabilitation for cardiovascular diseases and other chronic diseases by different medical associations worldwide. The guidelines for physical activity recommend regular and moderate exercise regimens. A meta-analysis found a significant improvement in all MetS components in patients who undertook lifestyle modifications compared to patients who maintained typical care 10. In addition to this study, another systematic review and meta-analysis revealed an overall positive effect of physical activity interventions on MetS parameters, suggesting that physical exercise may be an effective tool for MetS management 11.
Albuminuria control may be an effective measure for primary prevention of kidney diseases, cardiovascular mortality and overall mortality in the context of MetS. However, the effect of MetS treatment on reducing albuminuria (such as lifestyle changes, medication or surgical intervention) is inconclusive. Thus, the present study aimed to review the literature for evidence on the impact of available treatments on decreasing albuminuria in adult patients with MetS.
2. Methods
This was a systematic review of the literature followed by a meta-analysis. The protocol was registered in the Open Science Framework (OSF) platform (osf.io/g5b7a/), registered on the PROSPERO platform (ID: CRD42024523828) and published in a scientific journal 12. The present systematic review was conducted according to the Joanna Briggs Institute (JBI) methodology and the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) 13.
2.1 Data sources and searches
The present systematic review considered quantitative studies analyzing therapies used for MetS and their effects on reducing albuminuria in adult patients. The following databases were considered for the search: Cochrane, Embase, Scopus, Medline/PubMed and Dart-E Europe ETheses, without language restrictions. Studies from 2009 onward were included due to better standardization of the diagnostic criteria for MetS in 2009. The search was performed between August and September 2021. The following terms were used for the search strategy and adapted according to the database used: (Albuminuria; microalbuminuria; proteinuria; “metabolic syndrome”; “metabolic syndromes”; “metabolic syndrome*”; “insulin resistance syndrome”; metabolic*; “dysmetabolic syndrome*”; “Reaven syndrome*”; “metabolic cardiovascular syndrome”; “cardiometabolic syndrome”; “cardiometabolic syndromes”; therapeutics; therapeutical; therapeutically; therapeuticals; therapeutic; therapies; therapy; therapy s; therapys; therapeutics; treatments; treatment; and treatment s) using the Boolean operators OR and AND when applicable. The search strategy was established with the help of a librarian and was adapted according to the database used. As a supplementary strategy to retrieve new articles, a search was performed in the reference lists of the selected studies to identify additional studies.
2.2 Data selection
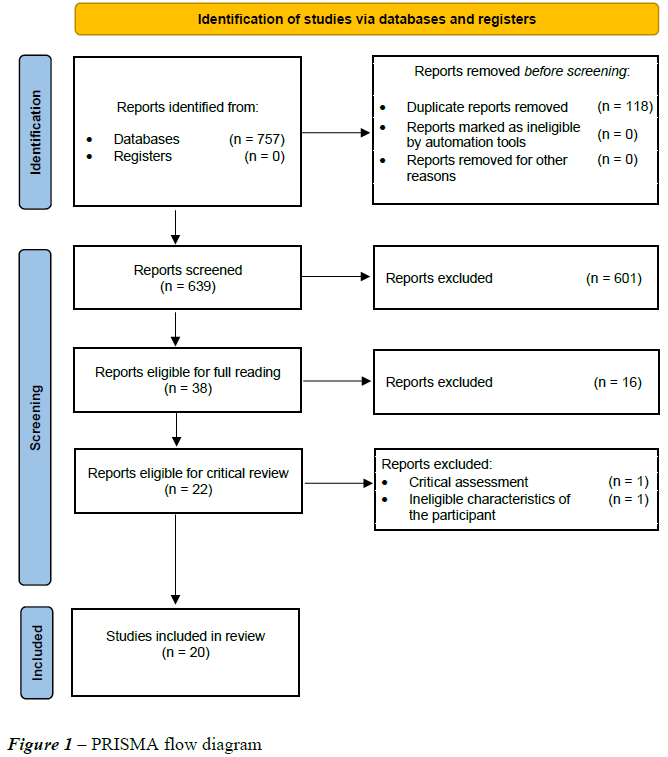
Studies were selected by two independent reviewers based on the inclusion criteria defined for the review. After the search, all identified records were grouped and sent to Mendeley (Mendeley Ltd., Elsevier, Netherlands), and all duplicate articles were removed. After reading the titles and abstracts of the studies retrieved for potential relevance, they were read and analyzed in full by the two reviewers. For studies in which the information was uncertain, the authors were contacted by e-mail. The complete study selection process is described in Figure 1.
2.3 Data extraction and quality assessment
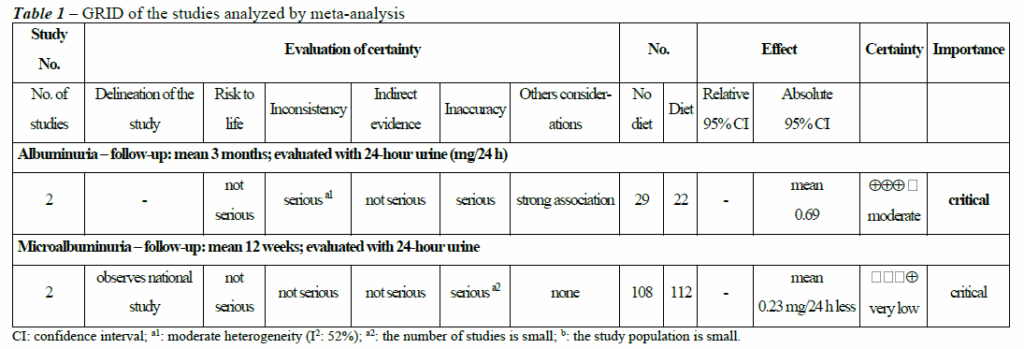
Data from the eligible articles were extracted independently by two researchers using a data collection instrument prepared by the authors in an Excel table containing the following information: authorship, study site, year of publication, type of study, sample size (n), MetS and albuminuria classifications, study objective, intervention applied, comparator, study results and limitations. Any discrepancies were corrected with the help of a third reviewer. Twentytwo studies were analyzed for methodological rigor using the JBI – SUMARI (System for the Unified Management of the Assessment and Review of Information) tool. Studies with scores equal to or greater than 70% of the items evaluated were included. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to determine the level of evidence for recommendations (Table 1).
2.4 Analyses
The data were organized and tabulated to answer the questions of the present review, and a narrative description of the data was developed such that the available evidence on the subject could be synthesized. The studies evaluating comparable treatment strategies and showing homogeneity in their methodological design were combined to perform the meta-analysis. To combine the continuous results of the studies, the Mantel‒Haenszel random effects model and differences between standardized means were used as measures of the effect. Additionally, the results of the I² statistical test were interpreted considering the magnitude and direction of the effect. Publication bias was not assessed due to the number of studies included. A forest plot was generated to present the results. The meta-analysis procedures were performed using Cochrane Review Manager software (RevMan, version 5.4.1).
2.5 Patient and public involvement
This is a systematic review of published results, with no patient participation or the need for an ethical assessment.
3 Results
A total of 757 studies were retrieved using the search strategy adopted in this review. After removing 118 duplicates and 601 articles that did not meet the inclusion criteria previously determined by reading the titles and abstracts, a total of 38 studies were considered eligible for full reading. Of these, 16 were excluded for not meeting the inclusion criteria. After a manual search of the references of the articles included in the review, no new articles were added, and 22 articles were ultimately included in the present review, one of which was excluded in the qualitative analysis and one in the final analysis because the populations assessed did not meet the inclusion criteria of this systematic review. A total of 20 articles were included. Twelve randomized controlled trials, two observational studies, two case‒control studies, two quasiexperimental studies and two cohort studies were included.
Among the articles analyzed, six (30%) evaluated changes in lifestyle, such as diet or physical exercise, to control albuminuria in the context of MetS. Only one study did not find results for albuminuria reduction in the diet group or in the diet and lifestyle change group 14-19.
Thirteen (65%) articles analyzed some drug or herbal medicine to control albuminuria in the context of MetS. Among the articles analyzed, 10 (76.9%) presented some type of positive result for microalbuminuria control, with decreased levels in the urine and statistical significance between the control and intervention groups. The following treatments categories were identified for patients with MetS in the literature reviewed: i) pharmacological treatments: angiotensin receptor blockers, aldosterone antagonists, amlodipine, ezetimibe, pitavastatin, cholestyramine and empagliflozin; ii) herbal medicines: Chinese herbs, Yiqi Huazhuo Gushen formula and soluble guar fiber gum; iii) nonpharmacological treatments: diet, physical exercise and diet associated with physical exercise; and iv) surgical treatment: bariatric surgery or gastric bypass surgery.
Three of the articles analyzed drugs from the category of angiotensin receptor blockers (23%), all of which showed a beneficial effect on albuminuria. Two articles evaluated aldosterone inhibitors (15.3%) and also showed a positive effect of this category of diuretics on albuminuria in the MetS population, with statistical significance between the groups evaluated.
In the present systematic review (5%) of the selected articles, we found a study evaluating bariatric surgery and subsequent weight loss and this strategy’s potential to decrease microalbuminuria (MA) 15, which declined for postoperative MA levels in 24-h urine (24-h urine albuminuria in mg/24 h: preoperative: 77.2 ± 124.8; at 18 months: 18.7 ± 13.4 (p < 0.05); at 24 months: 35 ± 23.9 with a nonsignificant p-value.
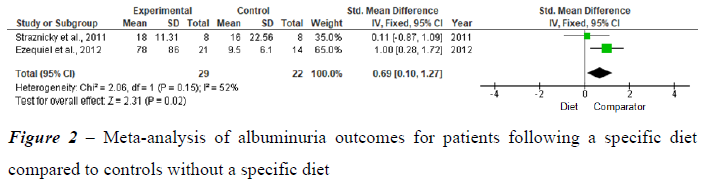
Three studies were analyzed by meta-analysis. The quantitative synthesis was performed by verifying the clinical and methodological homogeneity of the individual studies for the outcome diet compared to diet and exercise. Figure 2 shows the analysis of studies related to the impact of treatment on decreasing albuminuria in patients following a diet compared to controls without a specific diet.
Two studies were selected with outcomes related to changes in lifestyle (diet) as a therapeutic intervention for MetS that impacted albuminuria control. In the study by Straznicky et al. (2011), the group following the modified DASH diet was compared with the group without a specific diet. Albuminuria was reduced in the modified DASH diet group compared to the no-diet group. This finding was associated with weight loss and an increased glomerular filtration rate (GFR). In the study by Ezequiel et al. (2012), the effect of a hypocaloric diet (pre-post comparison) accompanied by a loss of at least 5% of the initial weight was analyzed. The authors observed a significant improvement in albuminuria control, which was associated with an improved lipid profile and an increased GFR. In the meta-analysis above, moderate heterogeneity was evident (I2: 52%), although substantial heterogeneity may have been present, with the significant p value revealing a trend for the diet group.
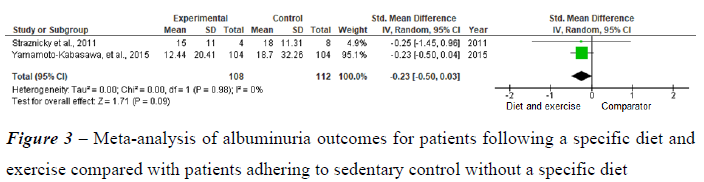
Figure 3 shows the analyses of the impact of diet in association with physical exercise on albuminuria. Straznicky et al. (2011), observed a reduction in albuminuria in the group undergoing a modified DASH diet in association with physical exercise compared to the group without a specific diet. This finding was associated with weight loss, an increase in the GFR, a decrease in blood pressure and decreases in C-reactive protein (CRP) and uric acid, with no change in blood glucose. Yamamoto-Kabasawa et al. (2015) compared the use of a diet in association with exercise with a sedentary lifestyle without a specific diet. Albuminuria was reduced with the diet followed by the physical exercise group compared to the sedentary group without a specific diet. This reduction was associated with weight loss, with no change in the GFR, decreased blood glucose in the group with DM and decreased blood pressure in the group with SAH.
Table 1 shows the GRADE types of the studies and effects on albuminuria. In the first evaluation, studies evaluating a diet group compared to a control group without a diet among patients with MetS and the associated effects on albuminuria were compared. The quality of the evidence of these studies corresponded to the degree of recommendation: moderate quality, Grade B, with the possibility that further studies may change the estimate of the effect and the confidence in it. In the second evaluation, studies evaluating diet in association with exercise compared with a sedentary lifestyle without a specific diet were compared. The quality of the evidence of these studies was considered very low, resulting in a degree of recommendation of Grade D, with the possibility that further studies may change the estimate of the effect and the confidence in the study.
4 Discussion
The current systematic review included 20 studies analyzing and comparing pharmacological and nonpharmacological treatments for MetS that could have an impact on albuminuria. The present study described the strategies used to control MetS that have an impact on decreasing albuminuria for the first time.
Regarding lifestyle changes, the present systematic review demonstrated that high-intensity aerobic exercise with or without resistance exercise was a promising intervention for reducing albuminuria in the context of MetS 18. Albuminuria, CRP, maximal oxygen consumption (VO2 max), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), high-density lipoprotein cholesterol (HDLc), leptin, resistin, interleukin 6 (IL-6) and waist circumference (WC) improved in the high-intensity aerobic exercise group and in the aerobic resistance exercise group. The improvement in albuminuria was accompanied by an improvement in glycated hemoglobin (HbA1C), decreases in interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNFα) and interferon gamma (IF-) and increases in the anti-inflammatory interleukins IL-4 and IL-10 only in the aerobic resistance exercise group 18. Nonpharmacological lifestyle change interventions are currently a well-established recommendation for patients with MetS. Several original articles, especially clinical trials, have investigated the impacts of a healthier diet on the components of MetS 8, 20.
In the current systematic review, a change in eating habits was also highlighted in some studies evaluated, and the following strategies were regarded as alternatives for MetS control with an impact on albuminuria: a hypocaloric diet with weight loss, a healthy diet (restricted in sugar and carbohydrates and rich in protein, vegetables and legumes) and a DASH diet based on foods rich in protein, fiber, potassium, magnesium and calcium, such as fruits and vegetables, beans, nuts, whole grains and low-fat dairy products, while limiting foods rich in saturated fat and sugars 14, 16, 17.
According to the present review, most (83.3%) of the studies evaluating lifestyle changes in patients with MetS showed promising results regarding the decrease in albuminuria with both diet and physical exercise, thus reinforcing the recommendation that changes in lifestyle constitute the basis of treatment for MetS, not only for the control of each of its components but also for the reduction in albuminuria when present 21. Although described as an important tool for MetS control and cardiovascular outcomes, no studies that evaluating the Mediterranean diet and its impact on albuminuria were found 6, 22, 23.
Despite the positive results for most diet types in the present systematic review, the most effective diet for decreasing albuminuria in this population could not be determined, which was mainly due to the high heterogeneity between the comparator factors and because several studies evaluated diets in association with exercise rather than in isolation. In addition, due to the low number of individuals analyzed in each study, the samples were heterogeneous; therefore, the results were described using metrics not statistically evaluated via meta-analysis (median; interquartile range). Thus, due to the high clinical and methodological heterogeneity of the studies evaluating the effects of different diet types on MetS, a meta-analysis of such data and comparisons of their effects on albuminuria were unfeasible.
Two meta-analyses were performed. The first meta-analysis included two studies and revealed that the intervention consisting of lifestyle changes (modified and hypocaloric DASH diets) in patients with MetS is associated with improved albuminuria control. This finding was associated with weight loss and an increase in the GFR. Weaknesses of the meta-analysis: only two studies were combined for the meta-analysis and had small sample sizes. The second metaanalysis included two studies with similar outcomes related to lifestyle changes (diet in association with physical exercise) in patients with MetS for albuminuria control, which showed improvements in albuminuria with both the modified DASH diet together with physical exercise and a hypocaloric diet together with physical exercise, both of which were associated with weight loss. Despite the decrease in albuminuria with both approaches, only the DASH diet in association with physical exercise showed improvement in the GFR. Weaknesses of the meta-analysis: Only two studies were combined for the meta-analysis, and they had small sample sizes, with severe imprecision among the studies evaluated. As shown in this meta-analysis, the practice of physical activity in association with a healthy diet may also be important for the consequences of MetS, such as albuminuria, and should be emphasized to all patients with MetS or risk factors for MetS.
The strength of the recommendation with this evidence was moderate because the benefits of diet and diet in association with physical exercise for reducing albuminuria in patients with MetS have been demonstrated, and the quality of the evidence in this study was considered to be moderate (Grade 2), with the possibility that further studies, preferably experimental studies with better methodological quality, may have an important impact on the estimate of the effect and the confidence in it.
Regarding pharmacological therapy, the positive impact of the use of renin-angiotensinaldosterone system inhibitors, aldosterone receptor blockers and aldosterone-inhibiting diuretics to decrease albuminuria in the diabetic population is well established in the literature 24-26, which was also demonstrated in the present systematic review in the population with MetS 27-31, and these drugs are considered the first-line treatment for albuminuria control in the general population. However, studies with comparisons between pharmacological and nonpharmacological interventions for MetS patients regarding the impacts on albuminuria control were not found.
Traditional drugs such as calcium channel blockers, ezetimibe and cholestyramine had a positive impact on albuminuria in patients with MetS, with the latter two being associated with an improved lipid profile in this population 29, 32, 33. Despite presenting promising results, these were isolated studies with small numbers of individuals, necessitating more clinical trials to evaluate the real benefit of these classes on albuminuria in MetS. In addition, no articles addressed the use of metformin or pioglitazone and their action on albuminuria in patients with MetS 34.
Empagliflozin, a sodium glucose transport protein 2 (SGLT-2) inhibitor, decreased mortality and chronic kidney disease progression in patients with MetS. Furthermore, a decrease in albuminuria was observed, which did not occur in the placebo group, and this pharmacological class is promising for future studies 35. Empagliflozin, a new drug class characterized by inhibition of sodium-glucose transport protein 2 (SGLT-2), has been presented in recent large clinical trials as a promising drug for the control of renal damage, albuminuria and cardiovascular mortality in diabetic patients 36.
Some herbal medicines have shown promise for albuminuria control in the study population, such as Chinese herbs, Yiqi Huazhuo Gushen formula and soluble guar fiber gum 31, 37, 38, but such studies were few and had small sample groups, and more clinical studies are necessary to evaluate the real benefit.
Only one study evaluated bariatric surgery with subsequent weight loss and its relationship with albuminuria 15, which declined for postoperative albuminuria levels; similarly, we found two studies directly evaluating weight loss due to changes in lifestyle and obtained similar results for albuminuria 14, 15.
The present study has some limitations that are worth noting. Most of the included studies had small sample sizes, which we considered a limiting factor in the evaluation of the effects of different approaches to MetS on albuminuria. Not all studies evaluated were randomized controlled trials, which is also a limitation of the present study and retrospective studies in general.
Limitations: The present systematic review did not establish a specific type of treatment, which led to high heterogeneity regarding the type of treatment, expected outcomes and especially the comparator factor, thus complicating quantitative analyses. Few studies were selected in the search, probably because the classification of MetS is defined by the presence of three or more components; therefore, patients may initiate treatment when they present only one or more factors. Articles exclusively investigating the treatment of only one of the components of MetS were not included.
Strengths: The present systematic review is the first to identify the positive impact of treatment on albuminuria for patients with MetS. In addition, it found that changes in lifestyle (diet and physical exercise) have been demonstrated to interfere with albuminuria control.
Furthermore, the studies support drugs with promising results in MetS.
5 Conclusion
A total of 20 articles were included, which revealed that lifestyle changes, angiotensin receptor blockers, aldosterone antagonists, herbal medicines and bariatric surgery seem to reduce albuminuria in patients with MetS. The meta-analysis included three studies and showed that diet alone or together with physical exercise is effective for reducing albuminuria in patients with MetS. In addition, the DASH diet, a hypocaloric diet and a diet associated with physical exercise have a positive impact on albuminuria control. However, no studies allowed comparisons between pharmacological and nonpharmacological treatments for albuminuria control in MetS patients.
Conflict of interest
All the authors declare that they have no conflict of interest with the contents of this article.
References
- Gargiulo P, Marsico F, Renga F, et al. The metabolic syndrome in heart failure: insights to specific mechanisms. . Heart Fail Rev. 2020;25(1):1-7. https://doi.org/10.1007/s10741-01909838-6
- Roomi MA, Mohammadnezhad M. Prevalence of metabolic syndrome among apparently healthy workforce. . J Ayub Med Coll Abbottabad. 2019;31(2):252–4. https://pubmed.ncbi.nlm.nih.gov/31094127/
- Rashidbeygi E, Safabakhsh M, Aghdam SD, et al. Metabolic syndrome and its components are related to a higher risk for albuminuria and proteinuria: evidence from a metaanalysis on 10,603,067 subjects from 57 studies. . Diabetes Metab Syndr. 2019;13(1):830–43. https://doi.org/10.1016/j.dsx.2018.12.006.
- Guzmán A, Navarro E, Obando L, et al. Effectiveness of interventions for the reversal of a metabolic syndrome diagnosis: an update of a meta-analysis of mixed treatment comparison studies. . Biomedica. 2019;39(4):647–62. https://doi.org/10.7705/biomedica.4684
- 5. Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. . Circulation. 2016;134(23):e535–e78. https://doi.org/10.1161/CIR.0000000000000450.
- Castro-Barquero S, Ruiz-León AM, Sierra-Pérez M, et al. Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients. 2020;12(10):E2983. https://doi.org/10.3390/nu12102983.
- Julibert A, Bibiloni MDM, Gallardo-Alfaro L, et al. Metabolic syndrome features and excess weight were inversely associated with nut consumption after 1-year follow-up in the PREDIMED-plus study. . J Nutr. 2020;150(12):3161–70. https://doi.org/10.1093/jn/nxaa289
- 8. Sayón-Orea C, Razquin C, Bulló M, et al. Effect of a nutritional and behavioral intervention on energyreduced Mediterranean Diet adherence among patients with metabolic syndrome: interim analysis of the PREDIMED-plus randomized clinical trial. JAMA. 2019;322(15):1486–99. https://doi.org/10.1001/jama.2019.14630.
- Tresserra-Rimbau A, Castro-Barquero S, Vitelli-Storelli F, et al. Associations between dietary polyphenols and type 2 diabetes in a cross-sectional analysis of the PREDIMED-Plus trial: role of body mass index and sex. . Antioxidants. 2019;8(11):1-17. http://dx.doi.org/10.3390/antiox8110537
- Marcos-Delgado A, Hernández-Segura N, Fernández-Villa T, et al. The effect of lifestyle intervention on health-related quality of life in adults with metabolic syndrome: a meta-analysis. . International Journal of Environmental Research and Public Health 2021;18(3):1-14. https://doi.org/10.3390/ijerph18030887.
- Sequi-Dominguez I, Alvarez-Bueno C, Martinez-Vizcaino V, et al. Effectiveness of mobile health interventions promoting physical activity and lifestyle interventions to reduce cardiovascular risk among individuals with metabolic syndrome: systematic review and metaanalysis. JMIR. 2020;22(8):1-17. https://doi.org/10.2196/17790
- Peralva BL, Santos KB, Lopes MGF, et al. Effect of clinical treatments for metabolic syndrome on albuminuria: a systematic review protocol. Rev Cienc Saude. 2023;13(1):22-5. https://doi.org/10.21876/rcshci.v13i1.1389
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):1-9. https://doi.org/10.1136/bmj.n71
- Ezequiel DGA, Costa MB, Chaoubah A, et al. Weight loss improves renal hemodynamics in patients with metabolic syndrome. Braz J Nephrol. 2012;34(1):36– 42. https://doi.org/10.1590/S0101-28002012000100006
- Kota SK, Ugale S, Gupta N, et al. Remission of type 2 diabetes mellitus by ileal interposition with sleeve gastrectomy. Int J Endocrinol Metab. 2011;9(3):374–81. https://doi.org/10.5812/Kowsar.1726913X.2782.
- Seligman BGS, Polanczyk CA, Santos ASB, et al. Intensive practical lifestyle intervention improves endothelial function in metabolic syndrome independent of weight loss: a randomized controlled trial. Metabolism. 2011;60(12):1736–40. https://doi.org/10.1016/j.metabol.2011.05.006
- Straznicky NE, Grima MT, Lambert EA, et al. Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J Hypertens. 2011;29(3):553–64. https://doi.org/10.1097/HJH.0b013e3283418875
- Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. . Nutr Metab Cardiovasc Dis. 2010;20(8):608–17. https://doi.org/10.1016/j.numecd.2009.04.015.
- Yamamoto-Kabasawa K, Hosojima M, Yata Y, et al. Benefits of a 12-week lifestyle modification program including diet and combined aerobic and resistance exercise on albuminuria in diabetic and non-diabetic japanese populations. . Clin Exp Nephrol. 2015;19(6):1079–89. https://doi.org/10.1007/s10157-015-1103-5
- Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. . Cell Metab. 2020;31(1):92-104. https://doi.org/10.1016/j.cmet.2019.11.004.
- Lemieux I, Després J-P. Metabolic syndrome: past, present and future. Nutrients. 2020;12(11). https://doi.org/10.3390%2Fnu12113501
- Bagetta D, Maruca A, Lupia A, et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur J Med Chem. 2020;186(2020):1-30. https://doi.org/10.1016/j.ejmech.2019.111903
- Martinotti S, Bonsignore G, Patrone M, et al. Mediterranean Diet polyphenols: anthocyanins and their implications for health. . Mini Rev Med Chem. 2021;21(13):1692–700. https://doi.org/10.2174/1389557521999201230200813
- Bakris GL, Agarwal R, Anker SD, et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. . Am J Nephrol. 2019;50(5):333–44. https://doi.org/10.1159/000503713.
- Delanaye P, Scheen AJ. Preventing and treating kidney disease in patients with type 2 diabetes. . Expert Opin Pharmacother. 2019;20(3):277–94. https://doi.org/10.1080/14656566.2018.1551362
- Jerums G, MacIsaac RJ. Treatment of microalbuminuria in patients with type 2 diabetes mellitus. Treat Endocrinol. 2002;1(3):163–73. https://doi.org/10.2165/00024677-20020103000004
- Haller H, Ito S, Jr JLI, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. . N Engl J Med. 2011;364(10):907– 17. https://doi.org/10.1056/nejmoa1007994
- Lovisi JCM, Ezequiel DAG, Costa MB, et al. Espironolactone improves flow-mediated vasodilatation in subjects with the metabolic syndrome. . Braz J Nephrol. 2011;33(4):463–6. https://doi.org/10.1590/S0101-28002011000400012
- Martinez-Martin FJ, Rodriguez-Rosas H, Peiro-Martinez, et al. Olmesartan/amlodipine vs olmesartan/hydrochlorothiazide in hypertensive patients with metabolic syndrome: The OLAS Study. . J Hum Hypertens. 2011;25(6):346– 53. https://doi.org/10.1038/jhh.2010.104.
- Suzuki H, Shuto H, Shuto C, et al. Eplerenone, an aldosterone blocker, is more effective in reducing blood pressure in patients with, than without, metabolic syndrome. . Ther Adv Cardiovasc Dis. 2012;6(4):141–7. https://doi.org/10.1177/1753944712452191
- Tian-zhan W, Qing-ping H, Bing W, et al. Synergistic effects of Yiqi Huazhuo Gushen herbal formula and valsartan on metabolic syndrome complicated with microalbuminuria. Trop J Pharm Res. 2019;18(1):101-8. http://dx.doi.org/10.4314/tjpr.v18i1.15
- Kato T, Inagaki K, Sawai Y, et al. Comparison of efficacy of pitavastatin and colestimide in japanese patients with diabetes mellitus complicated by hyperlipidemia and metabolic syndrome. . Exp Clin Endocrinol Diabetes. 2011;119(9):554–8. https://doi.org/10.1055/s-0031-1273770
- Yagi S, Akaike M, Aihara K-i, et al. Ezetimibe ameliorates metabolic disorders and microalbuminuria in patients with hypercholesterolemia. . J Atheroscler Thromb. 2010;17(2):173–80. https://doi.org/10.5551/jat.2378
- Larsen JR, Dima L, Correll CU, et al. The pharmacological management of metabolic syndrome. Expert Rev Clin Pharmacol. 2018;11(4):397–410. https://doi.org/10.1080/17512433.2018.1429910
- Ferreira JP, Verma S, Fitchett D, et al. Metabolic syndrome in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a post hoc analyses of the EMPA-REG OUTCOME Trial. Cardiovasc Diabetol. 2020;19(1):200. https://doi.org/10.1186/s12933-02001174-6.
- Piperidou A, Loutradis C, Sarafidis P. SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens. 2021;35(1):12–25. https://doi.org/10.1038/s41371-020- 00393-4.
- Dall’Alba V, Silva FM, Antonio JP, et al. Improvement of the metabolic syndrome profile by soluble fibre – guar gum – in patients with type 2 diabetes: a randomised clinical trial. . Br J Nutr. 2013;110(9):1601–10. https://doi.org/10.1017/S0007114513001025.
- Wang T-z, Chen Y, He Y-m, et al. Effects of chinese herbal medicine yiqi huaju qingli formula in metabolic syndrome patients with microalbuminuria: a randomized placebocontrolled trial. . J Integr Med. 2013;11(3):175–83. https://doi.org/10.3736/jintegrmed2013032
1Médica, Mestre em Saúde pela Universidade Federal de Juiz de Fora (UFJF). Juiz de Fora, Minas Gerais, Brasil
E-mail: babiperalva@yahoo.com.br ORCID: https://orcid.org/0000-0001-7613-2980
Nutricionista, Doutor em Saúde pela Universidade Federal de Juiz de Fora (UFJF). Empresa Brasileira de Serviços 2Hospitalares/Hospital Universitário da Universidade Federal de Juiz de Fora, Juiz de Fora, Minas Gerais, Brasil.
E-mail: marioflaviolima@gmail.com ORCID: https://orcid.org/0000-0002-5735-4411
3Professora associada II, Doutora em Saúde pela Universidade Federal de Juiz de Fora (UFJF). Faculdade de Enfermagem da Universidade Federal de Juiz de Fora, Juiz de Fora, Minas Gerais, Brasil.
E-mail: kelli.borges@ufjf.br ORCID: https://orcid.org/0000-0001-8423-9147
4Nutricionista, Doutora em Saúde pela Universidade Federal de Juiz de Fora (UFJF).
Hospital Unimed Dr. Hugo Borges, Juiz de Fora, Minas Gerais, Brasil.
E-mail: mguedes.nut@gmail.com ORCID: https://orcid.org/0000-0003-4035-8448
5Professor Titular, Doutor em Clínica Médica pela Universidade Estadual de Campinas (UNICAMP).
Núcleo de Pesquisa em Nutrição e Exercício Físico Aplicados à Síndrome Metabólica – NEFASM, Departamento de Clínica Médica. Faculdade de Medicina. Universidade Federal de Juiz de Fora, Juiz de Fora, Minas Gerais, Brasil.
E-mail: claudio.t.desouza@gmail.com ORCID: https://orcid.org/0000-0003-4904-5675
