REGISTRO DOI: 10.69849/revistaft/dt10202505112211
Lima, X,S.1; Portega, P,O.1; Roesler, C,R.1; Cavalcanti, A,S.2; Salmoria, G,V.1
ABSTRACT
Total hip arthroplasty (THA) is an effective procedure for relieving pain and restoring joint functionality. Despite advancements in ultra-high molecular weight polyethylene (UHMWPE) materials, such as cross-linking and vitamin E stabilization, aseptic loosening remains a major cause of implant failure, often associated with periprosthetic osteolysis. Submicron UHMWPE wear particles play a critical role in triggering inflammatory responses, leading to cytokine release and osteoclast activation. This narrative review analyzes literature from 2004 to 2024, focusing on the biological impact of submicron UHMWPE particles, their contribution to osteolysis, and aseptic losening. The findings highlight the need for continued material innovation to reduce inflammatory responses while maintaining durability.
Keywords: Ultra-high Molecular Weight Polyethylene – UHMWPE, Submicron wear particles, Inflammation, Debris.
1. INTRODUCTION
Total joint arthroplasty, including knee and hip replacements, is widely recognized as the gold standard for successful pain relief and restoration of joint function. Despite its success, since the introduction of ultra-high molecular weight polyethylene (UHMWPE) in joint prostheses, the long-term survival of implants in young patients remains limited by polyethylene wear (Stratton et al., 2022). Wear particles generated during the friction between the prosthesis’s tribological pairs lead to osteoclast-mediated bone resorption, periprosthetic osteolysis, and eventual aseptic loosening (Deans et al.,2023).
Over the past decades, advancements in materials such as UHMWPE and treatments to improve its wear resistance—including cross-linking, vitamin E addition, and thermal treatments—have resulted in greater wear resistance. However, aseptic loosening remains one of the main causes of arthroplasty failure, frequently associated with periprosthetic osteolysis. Periprosthetic osteolysis, triggered by prosthetic wear particles, is a major cause of aseptic loosening and subsequent revisions of hip implants. UHMWPE particles and other wear byproducts, continuously released from implant contact surfaces, generate a macrophage-mediated inflammatory response in periprosthetic tissue. This process initiates a cascade of events culminating in progressive bone loss around the implant, leading to loosening and eventual failure (Pajarinen et al., 2017).
Periprosthetic osteolysis is characterized by bone resorption around the implant, resulting in its loosening and eventual failure. This process is triggered by macrophage activation within the immune system in response to wear particles, which stimulate the release of pro-inflammatory cytokines such as TNF-α and IL-1. These cytokines, in turn, promote the differentiation and activation of osteoclasts, cells specialized in bone resorption. This inflammatory cycle results in significant bone loss and often necessitates surgical revisions, which are more challenging and have lower success rates compared to primary surgeries (Ingham & Fisher, 2005; Bozic et al., 2009).
Studies indicate that the size and concentration of UHMWPE particles play critical roles in macrophage activation. Submicron particles, in particular, have shown high biological activity. Matthews et al. (2001) observed that particles with an average size of 0.24 µm stimulate bone resorption at concentrations of 10 µm³/cell, whereas larger particles (0.45 µm and 1.71 µm) require higher concentrations (100 µm³/cell) to elicit similar effects. Similarly, Shanbhag et al. (1994) reported that particles in the size range of 0.1 to 1.0 µm are the most biologically active.
A 12-year study involving patients with hip implants made of conventional UHMWPE and cross-linked polyethylene (XLPE) found that seven hips in the conventional UHMWPE group exhibited osteolysis, while only one hip in the XLPE group presented osteolysis (P = 0.042). The authors concluded that, although the number of hips analyzed was insufficient to estimate causality for reduced wear in cross-linked UHMWPE, the study supports the hypothesis that HXLPE does not increase the risk of osteolysis. However, other studies suggest that HXLPE wear particles are smaller and thus more biologically active (Broomfield et al., 2017).
Although it is widely accepted that UHMWPE is the component most prone to wear, especially in hip and knee implants, there is no consensus in the literature regarding the shape, volume, or size of particles that most contribute to osteolysis and aseptic loosening, particularly regarding the immune system’s inflammatory response caused by submicron wear particles. This gap underscores the importance of reviewing and consolidating existing data to achieve a better understanding of this subject.
The objective of this narrative review is to provide a comprehensive overview of the literature published between 2004 and 2024, focusing on the relationship between submicron UHMWPE particles, the biological response they trigger, and the subsequent adverse effects, such as osteolysis and aseptic loosening.
2. MATERIALS AND METHODS
In this narrative review, the PubMed/MEDLINE, Web of Science, and Scopus databases were used as sources for searches conducted from October 5, 2024, to October 28, 2024, employing the following terms and criteria: “Submicron AND wear AND particles OR debris AND UHMWPE AND inflammation.”
Articles were selected based on the period from 2004 to 2024, in the English language, excluding review articles. Initially, a selection was made through the titles and abstracts of the articles. Those addressing the topic of interest were read in full by two researchers (SX and PP). Articles focusing on submicron wear particles of UHMWPE in implants and their relationship with osteolysis and/or aseptic loosening induced by biological activity were included. Studies that did not meet these criteria were excluded.
To observe the particle sizes studied in these articles and their respective results in terms of induction of the inflammatory process (biological activity), a table summarizing the relevant data was created.
3. RESULTS
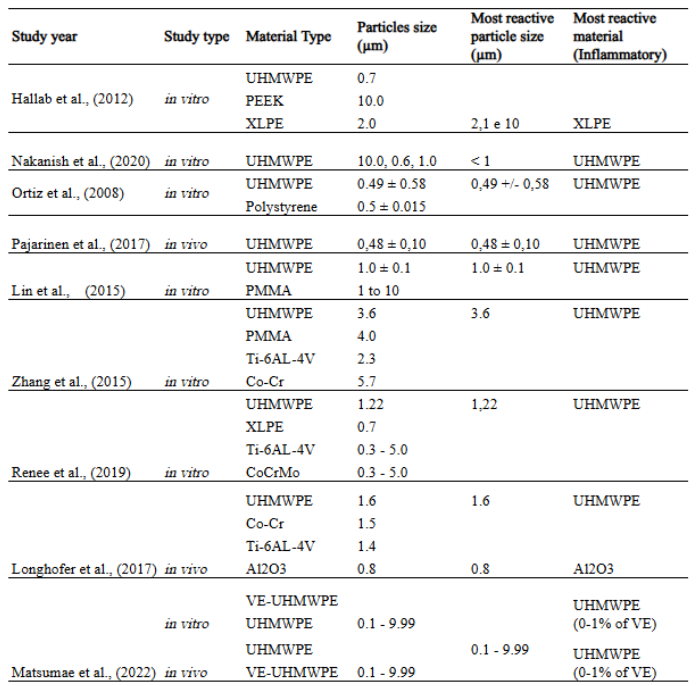
The initial search yielded 132 articles from Web of Science, 23 from PubMed/MEDLINE, and 4 from Scopus, totaling 159 articles. After analyzing the titles and abstracts, 24 articles were selected for full-text review. Of these, 13 were excluded for not meeting the inclusion criteria, resulting in 11 articles analyzed. A table 1.
Table 1: Relevant data on the type and size of wear particles in the biological response
I – Wear particles of UHMWPE
Renee et al. (2019) conducted a study to investigate the effects of wear particles from ultra-high molecular weight polyethylene (UHMWPE), highly cross-linked polyethylene (XLPE), and metal alloys (Ti6Al4V and CoCrMo) on human osteocyte-like cells derived from trabecular bone biopsies of patients who underwent total hip arthroplasty and experienced aseptic loosening. The polymer particles were produced and characterized by BioEngineering Solutions Inc. (Oak Park, IL, USA) from clinically relevant components using controlled methods, with average sizes of 1.22 µm (UHMWPE), 0.72 µm (XLPE), and 0.3–5 µm (metals). In vitro exposure of cell cultures revealed that both polyethylene and metal particles induced pro-inflammatory and pro-catabolic responses in osteocytes, suggesting their role in periprosthetic osteolysis and aseptic loosening.
The study demonstrated that polyethylene particles, particularly UHMWPE, significantly increased the expression of genes associated with osteoclastogenesis (RANKL, M-CSF) and osteocytic osteolysis (MMP13, CA2, and CTSK). Metal particles, particularly CoCrMo, showed even more pronounced effects on CA2 expression, highlighting the mineral dissolution capacity of osteocytes. Both types of particles also stimulated the production of pro-inflammatory cytokines (IL-6 and TNF-α) with different kinetics. These biological responses suggest that both osteocytes and osteoclasts participate in bone resorption processes induced by wear particles, with osteocytes playing a central role in matrix remodeling.
Interestingly, although XLPE was designed to reduce wear, it maintained similar or even higher bioactivity compared to UHMWPE in some cellular responses, such as the induction of MMP13, genes related to osteocytic osteolysis. Additionally, metal particles were associated with the activation of osteocyte apoptosis via caspase-3, while polyethylene did not exhibit this effect. Among the materials studied, UHMWPE particles exhibited the highest biological activity in promoting osteolysis, as they most strongly stimulated the expression of the pro-osteoclastic marker RANKL and induced elevated levels of genes related to osteocytic osteolysis, such as MMP13, CA2, and CTSK (ORMSBY, Renee T. et al., 2019).
Tzu-Hua Lin et al., (2015) aimed to evaluate other cytokines released in response to UHMWPE wear particles and polymethyl methacrylate (PMMA) particles, both with and without dendritic cells (DCs), given that Natural Killer T (NKT) cells, a subpopulation of T lymphocytes, can recognize self and foreign glycolipid antigens in the presence of antigen-presenting cells, including DCs. Once activated, these cells modulate the immune system by secreting pro-inflammatory cytokines such as interferon-γ (IFN-γ) or anti-inflammatory mediators such as IL-4.
Conventional UHMWPE particles were generated from a knee wear simulator, with an average diameter of 1.0 ± 0.1 µm, while commercial PMMA particles had an average diameter of 1–10 µm. It was observed that UHMWPE particles stimulated NKT cells to produce IFN-γ, particularly when co-cultured with DCs. These particles did not induce IL-4 secretion by NKT cells. NKT/DCs exposed to UHMWPE particles polarized bone marrow-derived macrophages (BMDMs) toward a pro-inflammatory M1 phenotype, increasing the expression of markers such as TNF-α and iNOS while reducing arginase-1 expression, a marker of the anti-inflammatory M2 phenotype. This effect was mediated by conditioned media from NKT/DC co-cultures, demonstrating that UHMWPE particles promote a complex inflammatory cascade involving cytokines secreted by macrophages and DCs.
These findings highlight the role of UHMWPE particles in activating unique immune pathways through interactions with NKT cells and DCs, resulting in exacerbated inflammatory responses and potential tissue damage. The increased pro-inflammatory activity of M1 macrophages, combined with cytokine secretion such as TNF-α, suggests that UHMWPE particles are critical mediators of periprosthetic osteolysis and that the stimulation of inflammatory cells related to osteolysis varies in intensity for different material types (LIN, Tzu-Hua et al., 2015).
Nakanishi et al. (2020) evaluated the biological response to UHMWPE particles using an experimental in vitro periprosthetic environment. To reduce the distance between macrophages and polyethylene particles, the authors developed a microfluidic device with a 200 × 500 µm channel. UHMWPE wear particles (GUR1050) were generated via pin-on-disk testing using UHMWPE pins and Co-28Cr-6Mo disks with varying surface finishes. The wear particles were collected in fetal bovine serum and filtered using membranes with pore sizes of 10 µm, 1.0 µm, and 0.6 µm. The authors reported that particles smaller than 1.0 µm were strongly associated with the release of inflammatory cytokines, such as TNF-α and IL-6, by human monocyte-derived macrophages. However, quantitative evaluation of osteolysis-related cytokine release could not be conducted under the conditions of this study.
Regarding the inflammatory reaction to the particles, the authors concluded that particles smaller than 1.0 µm resulted in greater secretion of inflammatory cytokines, such as TNF-α and IL-6, compared to larger particles. This study highlights the crucial role of particle size in the intensity of the inflammatory response, suggesting that smaller particles are more reactive and may contribute more significantly to periprosthetic osteolysis (NAKANISHI, Yoshitaka et al., 2020).
Ortiz et al., (2008) explored the chronic inflammatory processes induced by submicron-sized wear particles and their role in osteolysis using an in vitro mouse femoral explant model with continuous intramedullary particle infusion through osmotic pumps over two and four weeks. Polystyrene particles were commercially obtained (0.5 ± 0.015 µm in diameter – Polysciences, Warrington, PA), and UHMWPE particles with a mean diameter of 0.49 ± 0.58 µm were generated through a total joint wear simulation. In this study, UHMWPE particles were found to be more reactive than polystyrene particles, as evidenced by greater bone loss observed in mouse femurs exposed to UHMWPE particles at medium (2.60 × 10¹¹ particles/mL) and high (8.06 × 10¹¹ particles/mL) concentrations for two and four weeks, respectively.
Despite the high levels of bone loss observed with UHMWPE particles compared to the control group, which received only mouse serum infusion, this was associated with bone resorption. The authors noted that the model’s limitations prevented investigation of the cascade of events leading to bone resorption due to the absence of osteoclast trafficking at the affected site, an important step in particle-induced osteolysis (ORTIZ, Steven G. et al., 2008).
In the study by Pajarinen et al., (2017), a murine model was developed to investigate chronic osteolysis, replicating the clinical progression more accurately. Unlike in vitro studies that culture cells with the particles of interest to evaluate biological activity, the model proposed by the authors utilized continuous, slow delivery of UHMWPE particles through subcutaneous osmotic pumps, providing a steady flow of particles around an intramedullary implant. As a result, continuous delivery over eight weeks exacerbated bone loss and increased the presence of macrophages and peri-implant osteoclasts, offering a more precise simulation of the inflammatory response observed in patients with aseptic loosening.
The particles, generated using a knee joint simulator, had an average diameter of 0.48 ± 0.10 µm, derived from conventional UHMWPE. These findings highlighted the importance of considering both the quantity and characteristics of wear particles. In this study, the particles were spherical with an average diameter of 0.48 µm, reflecting a size within the biologically active range. The experimental environment in this work more accurately reproduced the inflammatory phenomenon caused by submicron wear particles and their impact on bone loss (PAJARINEN, Jukka et al., 2017).
Terkawi et al. (2018) conducted transcriptional analyses of human macrophages exposed to UHMWPE wear particles, revealing regulation of genes associated with cytokines, chemokines, and growth factors. Notably, Tumor Necrosis Factor Superfamily Member 15 (TNFSF15) and chemokine ligand 20 (CCL20) were identified as key molecules involved in inflammation and osteoclast activation.
The results showed that human macrophages exposed to UHMWPE particles, ranging in size from 0.1 to 10 µm (with 67.2% of particles within the 0.1–1 µm range), expressed genetic signatures common to rheumatoid arthritis, particularly those related to TNF receptor signaling cytokines. Additionally, the experiments demonstrated that TNFSF15, in combination with CCL20, significantly amplified osteoclastogenesis and bone resorption activity, suggesting that these molecules contribute to implant loosening. These findings emphasize the complexity of inflammatory and osteolytic responses associated with wear debris from orthopedic implants (TERKAWI, Mohamad Alaa et al., 2018).
II – Comparison of conventional UHMWPE with other materials
Kai Zhang et al. (2015) conducted a study comparing the biological activity of different materials, including PMMA, by investigating the impact of wear particles from different biomaterials used in joint prostheses—UHMWPE, PMMA, Ti-6Al-4V, and Co-Cr—on the activation of peripheral blood mononuclear cells (PBMCs) and associated inflammation. The average particle sizes studied were 3.6 µm for UHMWPE, 4.0 µm for PMMA, 2.3 µm for Ti-6Al-4V, and 5.7 µm for Co-Cr.
The results showed that UHMWPE particles induced the greatest infiltration of PBMCs into transplanted tissues, accompanied by elevated expression of chemokines such as MCP-1 and CXCR4 and pro-inflammatory cytokines such as IL-6. In contrast, Ti-6Al-4V particles were associated with higher osteoclastic activity, evidenced by TRAP staining, and elevated levels of TNF-α and IL-1β. PMMA particles exhibited intermediate effects, while Co-Cr particles induced more modest cellular responses. The authors noted that the group of monocytes activated by UHMWPE particles showed the highest level of cellular accumulation among all the biomaterial groups evaluated in this study, suggesting a greater potential to contribute to aseptic loosening and the failure of orthopedic implants. This study demonstrated that the composition and size of particles directly influence the severity of periprosthetic inflammation and osteoclastogenesis (ZHANG, Kai et al., 2015).
A similar study conducted by Longhofer et al. (2017) sought to understand the impact of different biomaterials—such as UHMWPE with an average diameter of 1.6 µm, cobalt-chromium (Co-Cr) with an average diameter of 1.5 µm, titanium alloy (Ti-6Al-4V) with an average diameter of 1.4 µm, and ceramic (Al₂O₃) with an average diameter of 0.8 µm—on the inflammatory and osteolytic response around implants. A murine model was employed to evaluate the particles of these biomaterials under an experimental protocol that eliminated mechanical factors, allowing the investigation of the biological effects of particles exclusively at the bone-implant interface.
In this study, 60 rats were divided into five experimental groups (UHMWPE, Co-Cr, Ti-6Al-4V, ceramic, and saline solution control) and underwent titanium rod implantation in the distal femur. Over a 16-week period, biomaterial particles were injected at the implant site biweekly, and bone mineral density (BMD) was monitored using micro-computed tomography (µCT). Histological and immunohistochemical analyses included the evaluation of osteoclastogenesis (marked by cathepsin K) and local inflammation, as well as the thickness of pseudomembranes formed at the bone-implant interface.
The results demonstrated that metal particles (Co-Cr and Ti-6Al-4V) caused the most significant reduction in periprosthetic bone density over the study period, suggesting a strong impact on bone resorption. However, these particles were not correlated with the highest levels of inflammation. Conversely, ceramic and UHMWPE particles induced more severe inflammatory responses, as evidenced by the significant thickness of pseudomembranes formed, although the effects on bone density were less consistent. Specifically, UHMWPE particles caused an acute reduction in bone density early in the study (at four weeks), followed by partial recovery. Ceramic particles promoted intense periprosthetic inflammation but had less direct impact on bone density.
The authors concluded that different biomaterials elicit distinct biological responses, influenced by both the composition and size of the particles. While metal particles were associated with sustained osteolysis, UHMWPE and ceramic particles showed greater capacity to stimulate local inflammation. These findings have direct implications not only for the development and design of materials but also for the selection of biomaterials aimed at minimizing osteolysis and inflammatory reactions (LONGHOFER, Lisa K. et al., 2017).
Hallab et al. (2012) compared the biological reactivity of UHMWPE particles, cross-linked UHMWPE (X-UHMWPE), and polyether ether ketone (PEEK-OPTIMA) particles of three sizes (0.7 µm, 2 µm, and 10 µm). UHMWPE and X-UHMWPE materials were obtained from sterilized acetabular cups (Enduron, Cat 1241-12-526, DePuy), and non-sterilized PEEK-OPTIMA implant-grade bars (PEEK-OPTIMA-LT1, Invibio Biomaterial Solutions) were used. Particles were tested on human THP-1 macrophages/monocytes and primary human monocytes over 24 and 48 hours. The authors observed that PEEK-OPTIMA and UHMWPE particles induced significant increases in cytokines IL-1β, IL-6, IL-8, TNF-α, and MCP-1 after 24 hours compared to unexposed controls (p < 0.05), with no significant differences after 48 hours. Cytokine responses to X-UHMWPE particles were significantly higher than those to other materials, with increases ranging from 4- to 100-fold compared to PEEK-OPTIMA.
The highest increase was observed for MCP-1, with values up to 10,000 pg/mL induced by X-UHMWPE particles of 2.1 µm, which was approximately 5,000 times greater than the controls and similarly sized PEEK-OPTIMA particles. Primary cell responses to PEEK-OPTIMA and UHMWPE particles also showed significant increases in IL-1β, IL-6, IL-8, and TNF-α for all tested individuals. In two of the three individuals, IL-1β responses to X-UHMWPE were higher than those to PEEK-OPTIMA. Larger particles of UHMWPE (13 µm) and X-UHMWPE (2.1 µm and 10 µm) induced significantly greater production of IL-1β, IL-6, IL-8, MCP-1, and TNF-α compared to PEEK-OPTIMA particles after 24 hours of exposure.
This study, despite its in vitro limitations, demonstrated that X-UHMWPE particles were more reactive than previously reported. Surprisingly, larger particles (>0.7 µm) of both X-UHMWPE and UHMWPE were more reactive. The authors concluded that PEEK-OPTIMA particles exhibited greater biocompatibility than UHMWPE particles, leading to reduced inflammatory cytokine production and suggesting that PEEK-OPTIMA implant debris presents a lower inflammatory risk than UHMWPE (HALLAB, Nadim James et al., 2012).
Another interesting study that evaluated the biological effects of UHMWPE wear particles with different concentrations of vitamin E (0.1%, 0.3%, 0.5%, and 1%) in in vitro and in vivo models, aiming to identify the optimal concentration to minimize inflammation and osteolysis, was conducted by Matsumae et al. The researchers used wear particles generated from medical-grade materials with predominant sizes ranging from 0.1 to 9.99 μm. In in vitro experiments, murine peritoneal macrophages stimulated with VE-UHMWPE particles showed significantly lower expression of TNF-α compared to conventional UHMWPE, regardless of the vitamin E concentration. In in vivo models, 0.3% VE-UHMWPE particles induced the lowest expression of TNF-α, IL-6, and RANKL in granulomatous tissues surrounding the particles, as well as the smallest areas of bone resorption, inflammatory cell infiltration, and TRAP staining.
As a conclusion of the study, the results suggest that VE-UHMWPE particles with 0.3% vitamin E have the lowest osteolytic and inflammatory potential, making this concentration the safest for extending the lifespan of implants, especially in young and active patients. However, 0.1% VE-UHMWPE particles induced osteolytic lesions comparable to those caused by conventional UHMWPE particles. These findings indicate that vitamin E can minimize the inflammatory effects caused by UHMWPE wear particles, provided that the particles contain a vitamin E concentration of 0.3%. (MATSUMAE, Gen et al., 2022).
In another in vivo study conducted by Huang et al. (2016), submicron-sized particles of different types of polyethylene were evaluated for their biological response. The study aimed to compare the biological response of UHMWPE, cross-linked polyethylene (XLPE), and vitamin E-doped highly cross-linked polyethylene (VE-HXLPE). The wear particles were produced using a micro-cutting process to achieve controlled sizes. The analysis revealed that XLPE particles had a smaller average size (0.47 ± 0.3 µm) compared to UHMWPE (0.71 ± 0.34 µm) and VE-HXLPE (0.61 ± 0.54 µm). Most of the generated particles were granular.
The results of the murine osteolysis model showed that XLPE caused the most intense osteolytic response, with significant reductions in bone mineral density (BMD), bone volume relative to tissue volume (BV/TV), and trabecular thickness (Tb.Th). This stronger response was attributed to the smaller average particle size, which resulted in a total particle quantity approximately three to four times higher for the same implanted weight, intensifying the inflammatory response.
Histological analyses confirmed a more severe inflammatory reaction in tissues treated with XLPE particles, characterized by soft tissue proliferation and increased osteoclast aggregation. Conversely, VE-HXLPE particles did not show significant differences compared to UHMWPE in terms of inflammatory or osteolytic response, suggesting that potential in vivo release of vitamin E did not negatively affect the osteolysis model.
The authors concluded that particle size and cumulative volume are critical factors in determining the intensity of the biological response, irrespective of the material type. Furthermore, while HXLPE demonstrated superior wear resistance, its smaller particle size could represent a heightened risk of long-term osteolysis. These findings underscore the importance of considering not only the material but also the behavior of wear particles in the development of biomaterials for prostheses (HUANG, Chang‐Hung et al., 2016).
4. DISCUSSION
The results of this narrative review highlight the complexity of the biological interactions mediated by submicron wear particles of UHMWPE. The intense pro-inflammatory and osteolytic activity of these particles is intrinsically linked to their size, material type, and morphological characteristics. Smaller particles (<1 μm) are more easily phagocytosed, resulting in greater macrophage activation and production of inflammatory cytokines such as TNF-α and IL-6 (Nakanishi et al., 2020; Terkawi et al., 2018).
Moreover, comparative analyses with other materials revealed that UHMWPE particles are consistently more reactive, inducing a robust inflammatory response and contributing to periprosthetic osteolysis. This behavior may be exacerbated by the lower wear resistance of conventional UHMWPE compared to XLPE and VE-UHMWPE, which demonstrate better mechanical performance but may have variable inflammatory implications. Additionally, XLPE wear particles induced cytokines and genes related to osteolysis, suggesting that submicron-sized particles play a critical role in driving the pronounced inflammatory response that leads to adverse events such as aseptic loosening (Huang et al., 2016; Matsumae et al., 2022; Pajarinen et al., 2017).
Furthermore, the choice of materials for the development of joint implants, as well as the selection of the material to be implanted in the patient, should consider not only mechanical resistance or biocompatibility but also biological activity and the inflammatory response.
5. CONCLUSION
This narrative review consolidated evidence that submicron wear particles of UHMWPE play a central role in inducing osteolysis and aseptic loosening of orthopedic implants. Their unique characteristics, such as size and composition, promote a complex inflammatory cascade involving pro-inflammatory cytokines and stimulating osteoclastogenesis. Despite advances in the development of more wear-resistant biomaterials, such as XLPE and VE-UHMWPE, biological reactivity remains a significant concern, considering that aseptic loosening continues to be one of the leading causes of revision surgeries.
The findings of this review emphasize the need for additional studies focusing on the submicron size of wear particles from materials commonly used in joint prostheses. These studies should aim to translate these findings into effective clinical applications and the development of materials that not only resist wear but also minimize the inflammatory response.
6. REFERENCES
STRATTON‐POWELL, Ashley A. et al. Mixed material wear particle isolation from periprosthetic tissue surrounding total joint replacements. Journal of Biomedical Materials Research Part B: Applied Biomaterials, v. 110, n. 10, p. 2276-2289, 2022.
DEANS, Christopher F.; BUCKNER, Brandt C.; GARVIN, Kevin L. Wear, osteolysis, and aseptic loosening following total hip arthroplasty in young patients with highly cross-linked polyethylene: a review of studies with a follow-up of over 15 years. Journal of Clinical Medicine, v. 12, n. 20, p. 6615, 2023.
PAJARINEN, Jukka et al. Murine model of progressive orthopedic wear particle-induced chronic inflammation and osteolysis. Tissue Engineering Part C: Methods, v. 23, n. 12, p. 1003-1011, 2017.
INGHAM, Eileen; FISHER, John. The role of macrophages in osteolysis of total joint replacement. Biomaterials, v. 26, n. 11, p. 1271-1286, 2005.
BOZIC, Kevin J. et al. The epidemiology of revision total hip arthroplasty in the United States. JBJS, v. 91, n. 1, p. 128-133, 2009.
MATTHEWS, J. B. et al. Comparison of the response of three human monocytic cell lines to challenge with polyethylene particles of known size and dose. Journal of Materials Science: Materials in Medicine, v. 12, p. 249-258, 2001.
SHANBHAG, A. S. Macrophage/particle interactions: Effects of size, composition and surface area. J. Bone Miner. Res., v. 8, p. 1071-1079, 1993.
BROOMFIELD, John AJ et al. The relationship between polyethylene wear and periprosthetic osteolysis in total hip arthroplasty at 12 years in a randomized controlled trial cohort. The Journal of arthroplasty, v. 32, n. 4, p. 1186-1191, 2017.
ORMSBY, Renee T. et al. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta biomaterialia, v. 87, p. 296-306, 2019.
LIN, Tzu‐Hua et al. Exposure of polyethylene particles induces interferon‐γ expression in a natural killer T lymphocyte and dendritic cell coculture system in vitro: A preliminary study. Journal of biomedical materials research Part A, v. 103, n. 1, p. 71-75, 2015.
NAKANISHI, Yoshitaka et al. Microfluidic device used for the secretion of inflammatory cytokines from human monocyte-derived macrophages stimulated by ultra-high molecular weight polyethylene particles. Biotribology, v. 23, p. 100137, 2020.
ORTIZ, Steven G. et al. Continuous intramedullary polymer particle infusion using a murine femoral explant model. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, v. 87, n. 2, p. 440-446, 2008.
TERKAWI, Mohamad Alaa et al. Transcriptional profile of human macrophages stimulated by ultra-high molecular weight polyethylene particulate debris of orthopedic implants uncovers a common gene expression signature of rheumatoid arthritis. Acta Biomaterialia, v. 65, p. 417-425, 2018.
ZHANG, Kai et al. Different influence of Ti, PMMA, UHMWPE, and Co‐Cr particles on peripheral blood monocytes during periprosthetic inflammation. Journal of Biomedical Materials Research Part A, v. 103, n. 1, p. 358-364, 2015.
LONGHOFER, Lisa K. et al. Specific material effects of wear-particle-induced inflammation and osteolysis at the bone–implant interface: A rat model. Journal of Orthopaedic Translation, v. 8, p. 5-11, 2017.
HALLAB, Nadim James et al. Macrophage reactivity to different polymers demonstrates particle size‐and material‐specific reactivity: PEEK‐OPTIMA® particles versus UHMWPE particles in the submicron, micron, and 10 micron size ranges. Journal of Biomedical Materials Research Part B: Applied Biomaterials, v. 100, n. 2, p. 480-492, 2012.
MATSUMAE, Gen et al. Determination of optimal concentration of vitamin E in polyethylene liners for producing minimal biological response to prosthetic wear debris. Journal of Biomedical Materials Research Part B: Applied Biomaterials, v. 110, n. 7, p. 1587-1593, 2022.
HUANG, Chang‐Hung et al. I n vivo biological response to highly cross‐linked and vitamin e‐doped polyethylene—a particle‐Induced osteolysis animal study. Journal of Biomedical Materials Research Part B: Applied Biomaterials, v. 104, n. 3, p. 561-567, 2016.
1Federal University of Santa Catarina – UFSC
2National Institute of Traumatology and Orthopedics – INTO
