REGISTRO DOI: 10.69849/revistaft/cl10202504151902
Lucas Bezerra Bandeira
Pedro Fernandes Vital
Alana Souza Holder
Fábio Fernandes de Oliveira Bispo
Guilherme de Sousa Diógenes
José Uilson de Sousa Pinheiro Junior
Ângelo Felipe Lima de Araújo Alves
Iasmim Gonçalves Henriques
Gabriel Diniz Câmara Dantas
Vinicius Silva Costa
1. Introduction
Type 1 Diabetes Mellitus (T1DM) represents an autoimmune-mediated disorder, resulting in destruction of the insulin producing pancreatic beta-cells which leads to progressive and irreversible failure of insulin secretion. T1DM accounts for about 10% of diabetes, affecting 1.4 million in the United States and about 15 million in the world1. Historically, T1DM was considered a disorder of children and adolescents, with incidence peaks occurring between 5–7 years of age, but the epidemiologic characteristics have changed largely by a plethora of environmental influences2. Genetic and clinical data varies in different countries and within the same country, mainly in regions like Brazil, where the population is one of the most heterogeneous in the world3. Adults with T1DM have a ten-fold increase in cardiovascular disease (CVD) risk and a two- to four-fold increased death rate attributed to CVD compared with the general population 4. Data on lipid and lipoprotein factors in youth with type 1 diabetes are scarce 5 although CVD is the leading cause of morbidity and mortality in T1DM 6. Therefore, the aim of this study was to evaluate available clinical parameters associated with the lipid profile as indirect markers of cardiovascular risk in a reference Endocrinology service of Northeast of Brazil.
2. Subjects, materials and Methods
This cross-sectional study included 67 patients with T1DM, diagnosed according to the American Diabetes Association criteria7, in patients that needed insulin for blood glucose control at or shortly after diagnosis and used continuously thereafter. These patients had been in regular follow-up in our endocrinology service of the University Hospital. The following data were recorded for each patient: age, sex, ethnicity, time of diagnosis, body mass index (BMI), presence of other autoimmune diseases, hypertension treatment and retinopathy. Laboratory evaluation included the measure of glycosylated hemoglobin (HbA1c), fasting blood glucose (FBG) total cholesterol (TC), low density lipoprotein (LDL), high density lipoproteins (HDL) and triglycerides (TG).
3. Statistical analysis
Baseline characteristics of the patients were expressed in mean ± standard deviation (SD) and percentage. It was used the Pearson correlation coefficient (r) and for TG data, the Spearman correlation coefficient (rs). Statistics were pre-configured at a confidence interval (CI) of 95% and significance of p ≤0.05.
4. Results
4.1 Demographic e clinical data
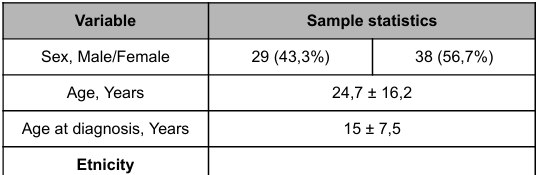
The baseline characteristics of the patients are shown in Table 1. The 67 patients included 38 females (56,7%) and 29 males (43.7%), with mean age of 24.7 ± 16.2 years. The mean age at diagnosis was 15 ± 7,5 years. The Brazilian population was formed by a strong admixture from different ancestral roots and, therefore, the ethnic identification was based predominantly on skin color. Brown color was the most prevalent, corresponding to 51 patients (78,4%), followed by white population with 13 patients (20%) and the black color with 1 patient (1,6%).
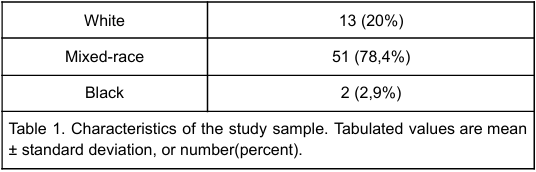
In Table 2 it is possible to perceive that three patients (4,5%) had hypothyroidism and other autoimmune diseases were present in 5,9 % of patients (two with celiac disease, one with vitiligo e one case of Addison disease). Also, seven patients presented with hypertension (10,5%) and 15 (22.4%) patients had albuminuria, of which 10 (14,9% of the total sample) developed moderately increased albuminuria and 4 (6%) developed severely increased albuminuria. Diabetic retinopathy was present in 8 (12%) patients.
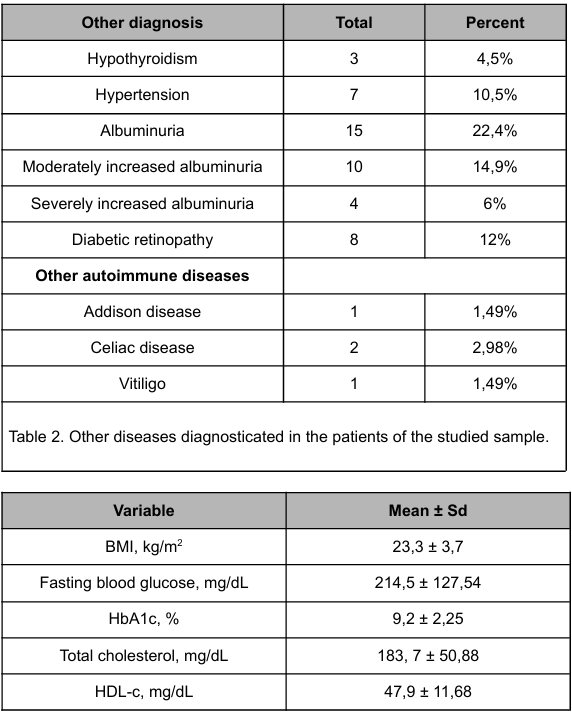
Table 3 presents the mean values of BMI, HbA1c, FBG, TC, HDL, LDL and TG were respectively, 23.3 kg/m² ± 3.7; 9.2% ± 2.25; 214.5 mg/dL ± 127.54; 183.7 mg/dL ± 50.88; 47.9 mg/dL ± 11.68; 112.3 mg/dL ± 40.66; 111.3 mg/dL ± 61.9.
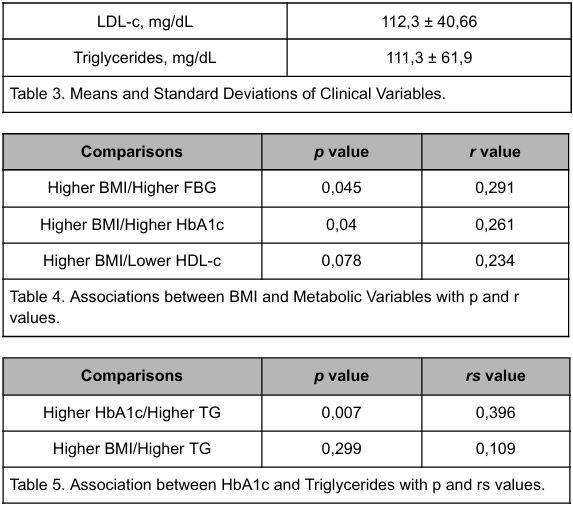
According to statistics analysis that are present in tables 4 and 5, there is a correlation between higher BMI and higher FBG (p=0.045; r=0.291) and higher BMI and higher HbA1c (p=0.04; r=0.261). Also, there was a correlation between HbA1c and TG, in which poor glycemic control was associated with higher TG (p=0.007; rs= 0.396). However, there was not an association between BMI and TG (p=0.299; rs=0.109) and neither between BMI and the HDL data (p =0.078; r=0.234).
5.0. Discussion
Dyslipidemia is a metabolic abnormality commonly found in young patients with T1DM, rising the cardiovascular risk in this population8 and shortened life expectancy, especially after 20 years of illness, as evidenced in the Diabetes Control and Complications Trial (DCCT) and the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study9. The UK General Practice Research Database (GPRD), comprising data from 7.400 patients with T1DM, demonstrated that these individuals had cardiovascular disease 10-15 years earlier than in matched nondiabetic control subjects10. In pathophysiological terms, there was a vascular-wall inflammation and an increased circulating inflammatory markers11, often associated with insulin resistance and metabolic syndrome which lead to vascular dysfunction, independent of glycemia12.
With regard to LDL-C in T1DM, the smallest particle size and the multiple enzymatic and non-enzymatic modifications that occurs due to hyperglycemia are the factors postulated for which this lipoprotein increase the risk of atheroesclerotic disease13. In this cohort of 67 T1DM patients, most patients had LDL levels above the target (> 100mg/dl) according the american diabetes association recommendations, clearly showing the importance of lipid evaluation for these patients14. Our results are corroborated by other Brazilian studies, which showed average LDL values above 100mg/dl 15,16.
Despite the scarcity of studies on lipid-lowering therapy in patients with type 1 diabetes, the Swedish National Diabetes Register showed a significant reduction in several cardiovascular outcomes, over a 6-year follow-up, within the group that used statin. 17 .
There was a positive correlation between HbA1c and TG in our study, however, there was not an association between BMI and TG and neither between BMI and the HDL. Such findings can be explained by the fact that insulin therapy can, at the same time, lead to weight gain and a reduction in triglycerides, as there is a greater stimulation of lipoprotein lipase that degrades particles rich in triglycerides and inhibition of hormone-sensitive lipase 18. In our study sample, the average triglyceride value was 111.3 mg/dL ±61.9, therefore, within the target recommended by the guidelines.
Regarding the influence of race, there are some studies that have shown an increase in cardiovascular risk in people with T1DM of black color 19,20. Our population was formed by a strong admixture from different ancestral roots, making it difficult to establish a causal relationship linked to race, but what is known is that ethnic differences can act on genetic or biological factors, in addition to the influence of other comorbidities, such as hypertension, whose prevalence differs between races.
Patients with T1DM represent a population with high risk for developing cardiovascular diseases and early mortality. However, because they are young, dyslipidemia treatment is often postponed in clinical practice, which contradicts with our study and others that demonstrates high cholesterol levels, many of which require treatment.
6. References
- Daga, RA t al. Demographic and Clinical Characteristics of Diabetes Mellitus among Youth Kashmir, India. Int J Pediatr, Vol.3, N.4-1, Serial No.19, Jul 2015
- Gomes, KFB. The influence of population stratification on genetic markers associated with type 1 diabetes. Sci Rep. 2017; 7: 43513.
- Mattan, TCC. CD226 rs763361 Is Associated with the Susceptibility to Type 1 Diabetes and Greater Frequency of GAD65 Autoantibody in a Brazilian Cohort. Mediators Inflamm. 2014; 2014: 694948.
- Risk Factors for Cardiovascular Disease (CVD) in Adults with Type 1 Diabetes: Findings from Prospective Real-life T1D Exchange Registry. Jcem
- Lipid and Lipoprotein Profiles in Youth With and Without Type 1 Diabetes John Guy, Lorraine Ogden, R. Paul Wadwa, Richard F. Hamman, Elizabeth J.Mayer-Davis, AngelaLiese, Ralph D’Agostino, Santica Marcovina, Dana Dabel ea Diabetes Care Mar 2009, 32 (3) 416-420; DOI: 10.2337/dc08-1775
- Hartz JC, de Ferranti S, Gidding S. Hypertriglyceridemia in Diabetes Mellitus: Implications for Pediatric Care. J Endocr Soc. 2018 May 1;2(6):497-512. doi: 10.1210/js.2018-00079. PMID: 29850649; PMCID: PMC5961027.
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. American Diabetes Association.Diabetes Care 2019 Jan; 42(Supplement 1): S13-S28.
- Zabeen B, Balsa AM, Islam N, Parveen M, Nahar J, Azad K. Lipid profile in relation to glycemic control in Type 1 diabetes children and adolescents in Bangladesh. Indian J Endocr Metab 2018;22:89-92
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care 2016; 39:1378-83.
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 2006;29:798–804
- Janssen AWM, van Heck JIP, Stienstra R, et al. Arterial wall inflammation assessed by 18F-FDG-PET/CT is higher in individuals with type 1 diabetes and associated with circulating inflammatory proteins. Cardiovasc Res 2023;119: 1942-51.
- Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26:1374-9.
- Modified Low Density Lipoprotein and Lipoprotein-Containing Circulating Immune Complexes as Diagnostic and Prognostic Biomarkers of Atherosclerosis and Type 1 Diabetes Macrovascular Disease. Alexander N. Orekhov 1,2, Yuri V. Bobryshev 1,3,4,*, Igor A. Sobenin 1,5, Alexandra A. Melnichenko 1 and Dimitry A. Chistiakov. Int. J. Mol. Sci. 2014, 15, 12807-12841.
- Children and Adolescents: Standards of Medical Care in Diabetes−2020. American Diabetes Association. Diabetes Care 2020 Jan; 43(Supplement 1): S163-S182.
- Arcanjo CL, Piccirillo LJ, Machado IV, Andrade CRM, Clemente EL, Gomes MB. Avaliação de dislipidemia e de índices antropométricos em pacientes com diabetes mellitus tipo 1. Arq Bras Endocrinol Metab. 2005;49(6):951-8.
- Castro SH, Castro-Faria-Neto HC, Clemente ELS, Gomes MB. Avaliação da suscetibilidade do LDL de pacientes com diabetes mellitus tipo 1 à oxidação in vitro e a sua relação com o controle glicêmico. Arq Bras Endocrinol Metabol 2004:48(4):513-7
- Hero C, Rawshani A, Svensson A-M, et al. Association Between use of lipidlowering therapy and cardiovascular diseases and death in individuals with type 1 diabetes. Diabetes Care 2016;39: 996-1003.
- Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989 Apr 20;320(16).
- Roy M, Rendas-Baum R, Skurnick J. Mortality in African-Americans with type 1 diabetes: the New Jersey 725. Diabet Med 2006;23:698– 706
- Bosnyak Z, Nishimura R, Hagan Hughes M, et al. Excess mortality in black compared with white patients with type 1 diabetes: an examination of underlying causes. Diabet Med 2005; 22:1636–1641
