REGISTRO DOI: 10.69849/revistaft/th102501091202
Antonia Lúcia dos SANTOS1
Vilma dos SANTOS 2
Felipe Schwahofer LANDUCI3
Rennan Ribeiro da SILVA4
Rafael Fonseca HEINRICHS4
Tatiana Dillenburg SAINT’PIERRE5
Salvatore Giovanni DE SIMONNE1
Rachel Ann HAUSER-DAVIS6
Abstract
Molluscs can bioaccumulate infectious microorganisms and potentially toxic chemical elements, which result in high mortality rates and in negative human health effects, threatening the entire production chain. In Brazil, the largest native scallop (Nodipecten nodosus) productions are located in southeastern and, although displaying great socioeconomic importance, no environmental or sanitary monitoring plan is established in this region. In this context, this study aimed to carry out preliminary chemical assessments in cultivatedlion’s paw Nodipecten nodosus scallops in southeastern Brazil. Metals and metalloids are in similar levels of those registered in the same area by other authors and lower than registered in other cultivation sites around the world. All THQ values were below 1, indicating that it is unlikely that consumers will experience obvious adverse effects from the consumption of scallops from the sampling location
This type of assessment in high-production regions can aid in establishing sanitary control measures, focusing both on the scallop production chain and Public Health concerns.
Keywords: Public Health; metal contamination; mariculture; miticulture; scallops.
INTRODUCTION
Coastal environments worldwide have become chronically polluted due to rapid industrialization and urbanization processes and inadequate sewage and wastewater treatments, affecting the sanitary condition of food produced in these areas (Capillo et al., 2018;Lin et al., 2021). Particularly in the South Atlantic, anthropic activities result in direct pollutant discharges into aquatic environments, including mangroves, bays and estuaries, from the continent, such as runoff, industrial effluents, mining and agriculture, as well as a lack of basic sanitation, while others originate from activities such as navigation, fishing and aquaculture and oil exploration, in addition to direct atmospheric deposition (Hatje et al., 2021). This may, in turn, directly affect the quality of seafood used for human consumption, which can lead to public health issues (Pereira et al., 2007)
Molluscs in general display the capacity to accumulate potentially toxic contaminants and pathogens, such as metals and metalloids (Galvão et al., 2009), bacteria (Pereira et al., 2007) viruses (Wittman and Flick, 1995;Potasman et al., 2002;Leal and Franco, 2008;Arzul et al., 2017), protozoa (Lévesque et al., 2010) and saprophytic fungi (Sallenave-Namont et al., 2000), in their tissues due to their filter-feeding habits (Lin et al., 2021).
Belonging to the Pectinidae family, scallops are of enormous economic importance in several countries, such as China, Peru, Chile, Japan, Korea, the United States, France, Canada, England, Spain, and Phillipines. Chile and Peru are the largest producers in Latin America ((FAO, 2020; Kluger et al., 2019). In Brazil, a small but growing production of lion’s paw scallops (Nodipecten nodosus) is also noted, mainly in the states of Rio de Janeiro (RJ), São Paulo (SP) and Santa Catarina (SC) (Valenti et al., 2021).
Rio de Janeiro is the second most important state in the country in terms of number of inhabitants, population density and gross domestic product (IBGE, 2021). The state has developed and consolidated marine aquaculture over the past three decades as a productive economic activity of great local and regional relevance. Scallop cultivation remains the main activity in terms of total production and number of farmers, but new challenges and concerns have now emerged (Landuci et al., 2021). These include a mortality outbreak, a lack of information concerning the hygienic-sanitary conditions and environmental characteristics of molluscs farms and their vulnerability to contamination by metals and metalloids and different pathogens, that can lead to high mollusc mortality rates and threaten the mollusc chain production (Stewart et al., 2021). Also, this generates a vulnerability of the productive sector with regard to food security (Souza et al., 2015), and makes it impossible for products to access the international market (FAO and WHO, 2018;Kluger et al., 2019), requiring action by the public sector. Because of this, it is important that all specific hazards in the mollusc production chain, such as pathogens and chemical pollutants, compounds be identified.
Ory et al. (2021) reports a diversity of interactions between metals and the complexity of the physiological response of marine bivalves to pollutants. For example, Zn exposure for 48 h in the variegated scallop (Mimachlamys varia) was shown to alter several biological processes, such as energy metabolism, osmoregulation, defense against oxidative stress and purine and tryptophan metabolism. In another assessment, Sobrino-Figueroa et al. (2007) demonstrated that Catarina scallop (Argopecten ventricosus) juveniles are more sensitive to Cd, Cr and Pb compared to other marine species and under certain conditions, a mixture of these metals produces more toxic effects where Cd exhibited a higher effect on the growth rate of A. ventricosus juveniles and decreased the growth rate of A. ventricosus 64- and 5-fold lower than the concentrations observed in other species, such as as A. irradians and Nassarius festivus, respectively.
Furthermore, clear differences in metal toxicity in different scallop life stages are also reported (Romero-Murillo et al., 2017). For example, A. purpuratus embryos were shown to be more sensitive to Pb than most other bivalve species, while juvenile scallops exhibited an opposite pattern concerning metal toxicity compared to embryos, of Cd being more toxic than Pb.
In this context, the aim of this study was to evaluate the concentrations of various metals and metalloids in lion’s paw scallops destined for commercialization for human consumption collected from mariculture farms located in the state of Rio de Janeiro, Southeastern Brazil.
MATERIAL AND METHODS
Scallop sampling and processing
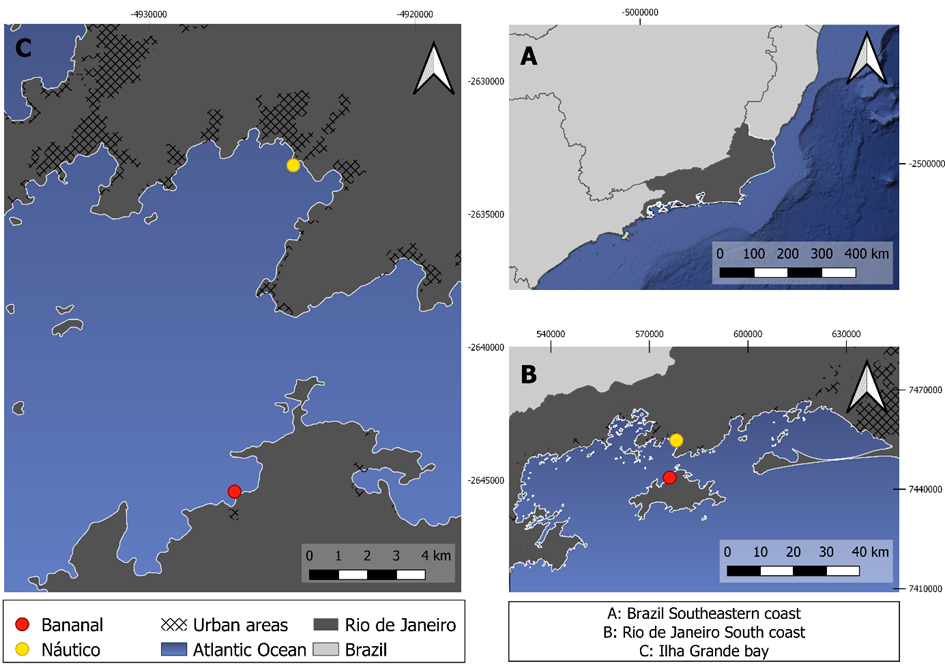
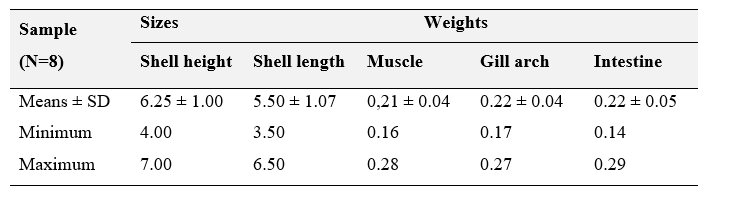
Adult lion’s paw scallop individuals (n=8) averaging a shell lenght size of 6.25 ± 1 cm were sampled from a marine farm in Bananal cove (Fig, 1), in Ilha Grande Bay, Angra dos Reis, in the state of Rio de Janeiro. The samplings were carried out manually at random in January 2018, right after the notification of an outbreak of mortality from unidentified causes. The samples were washed with sea water at their place of origin, packed in isothermal boxes and transported for 12 km to the Ilha Grande Bay Ecodevelopment Institute (IEDBIG). The scallop valves were opened with the aid of a sterile spatula and with muscle, gill arch and intestine samples were removed with a scalpel and sterile forceps, and freeze until analyses. Only few scallops could be collected due the low survival recorded after the mortality outbreak
Fig 1. Lion’s paw scallop sampling site at Jaconema Beach, Angra dos Reis, southeastern Brazil.
Metal and metalloid determinations
A total of 100 mg of tissue samples were weighed on an analytical balance in sterile 15 mL screw-capped polypropylene tubes. Acid decomposition was performed overnight by adding 1.0 mL of double distilled HNO3 (Hexis, Rio de Janeiro, Brazil) to each sample and closing the tubes tightly. The next day, the samples were heated for four hours at 100 ºC in a heating block in the closed tubes to avoid loss of volatile elements (USP, 2013). After cooling, the samples were made up with 10 mL of Milli-Q water (resistivity > 18.0 MΩ cm) obtained using a Merck Millipore water purification system (Darmstadt, Germany) and analyzed by inductively coupled plasma mass spectrometry (ICP-MS) employing a NexIon 300X mass spectrometer (PerkinElmer, Norwalk, CT, USA) according to the USEPA 6020B method (EPA, 2014). External multi-element calibration curves were prepared and 102Rh was used as an internal standard introduced online during the entire analysis. All correlation coefficients of the analytical curve were above 0.995. Blanks and ERM®-BB422 certified reference material (fish muscle, European Commission) were prepared in the same way as the samples to ensure method accuracy and precision.
Digestive gland could accumulate metals via ingestion and gill arch was considered as an initial owing to its large surface area (Xu et al., 2020). In molluscs, gills are always in direct and continuous contact with metals in the dissolved aqueous phase of the aquatic environment which leads to contaminant accumulation at considerably faster rates in this tissue compared to other organs, resulting in gill metal storage (Hauser-Davis et al., 2021a). The adductor muscle, on the other hand, although it does not have a large accumulation compared to the other organs, is the main edible part of the scallop.
All samples, blanks and certified reference materials were analyzed in triplicate. The limits of quantification (LOQ) for each determined element were calculated according to the National Institute of Metrological Standards and Industrial Quality (Inmetro), as LOQ = (10 * SD * df)/slope of the line, where SD comprises the standard deviation between the ratio of the analytical signal to the internal standard signal of 10 blank solutions and df is the dilution factor applied to the sample (Inmetro, 2020). The LOQs of each determined element were as follows: Al 0.220; At 0.018; Cd 0.002; Co 0.002; Cr 0.310; Cu 0.042; Fe 1310; Hg 0.036; Mn 0.005; Ni 0.019; Pb 0.004; If 0.140; Ti 0.160; V 0.110; Zn 0.270 mg kg-1 wet weight.
Estimated weekly intake
The estimated weekly intake (EWI) was calculated according to Equation 1:
EWI = (C×MWI)/BW Equation 1
Where EWI is the estimated weekly intake, C is the mean element concentration, MWI is the mean shellfish intake, and BW is the adult body weight. The average fish and seafood consumption values in Brazil was used as a basis for this calculation(FAO, 2020). The annual consumption of molluscs (excluding cephalopods) corresponds to about 0.2 kg. The means for each element in each tissue were multiplied by the average weekly consumption (3.84 g) and divided by the average body weight of a Brazilian adult (70 kg). The results were compared to the provisional tolerable weekly intake (PTWI) and maximum allowable limit (MPL) values provided by various regulatory agencies.
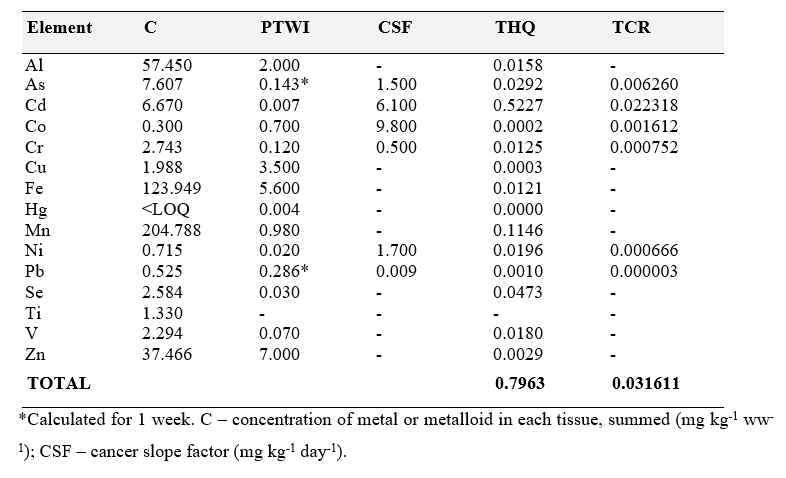
THQ (target risk quotient) and TCR (target cancer risk)
General and specific cancer risk factors were calculated for each of the elements evaluated in the scallops, according to Equations 2 and 3:
THQ= (EFR×ED×FIR×C) / (PTWI×BW×ATn)×10−3 Equation 2
TCR= (EFR×ED×FIR×C×CSF) / (BW×ATc)×10−3 Equation 3
Where EFR – frequency of exposure to the metal or metalloid (365 days); Ed – duration of exposure (average 70 years of life); FIR – intake rate (3.84g/week); C – concentration of metal or metalloid in each tissue, summed; BW – body weight (considering a 70 kg adult); AT – average exposure time (25,550 days); CSF – cancer slope factor (mg kg-1 day-1). The THQ describes the non-cancer health risk posed by exposure to the respective element. If the THQ is <1 non-carcinogenic health effects are not expected. If, however, the THQ is > 1, there is a possibility of adverse health effects. A THQ greater than 1, however, is not a statistical probability that non-cancerous adverse health effects will occur. The THQ was estimated using the United States Environmental Protection Agency methodology (US-EPA, 1989) based on the Region III risk-based concentration table. The TCR is used to assess the potential risk associated with exposure to carcinogens in the lifetime exposure period. A cancer propensity factor by ingestion is used that determines, along with the carcinogen dose, the likelihood of excess cancer risk over the lifetime of the exposed individual(US-EPA, 2000).
Statistical analyses
All statistical analyses were performed using the GraphPad Prism v.9 software. Data normality was verified by the Shapiro-Wilk test. As metals and metalloids did not display a normal distribution, non-parametric tests were performed. To assess potential significant differences in the levels of metals and metalloids between the evaluated tissues, the Kruskal-Wallis test was applied. A value of p < 0.05 was accepted as statistically significant for the comparisons between all groups, and p < 0.01 for the pairwise comparisons of the evaluated tissues. The correlations between the levels of metals and nonmetals and the total length of the scallops were evaluated by Spearman’s correlation test, employing the following correlation strengths: 0.00 <r <0.19 – very weak; 0.20 <r < 0.39 – weak; 0.40 < r < 0.69 – moderate; 0.70 <r <0.89 – strong and 0.90 <r <1.0 – very strong (Bryman and Cramer, 2011). Only moderate, strong and very strong correlations were considered and discussed.
RESULTS
The biometric information of the analyzed scallops is presented in Table 1. The sizes of the scallops are presented in centimeters (cm) and the weights, in grams (g).
Table 1. Morphometric data for the Nodipecten nodosus scallops analyzed herein (N=8). Sizes are presented as centimeters (cm) and weights, as grams (g).
All determined metal and metalloid concentrations for each analyzed Nodipecten nodosus scallop tissue are presented in Table 2. Values are expressed as means ± standard deviations. Mercury was below the limit of quantification for all sampled tissues
Table 2. Metal and metalloid concentrations (mg kg-1 ww-1) in Nodipecten nodosus (N=8) scallop tissues analyzed herein. Values are expressed as means ± standard deviations. Significant differences between tissues for each element are indicated in superscript letters, where the same superscript letter in different tissues indicates a statistically significant difference by the Kruskal-Wallis test.
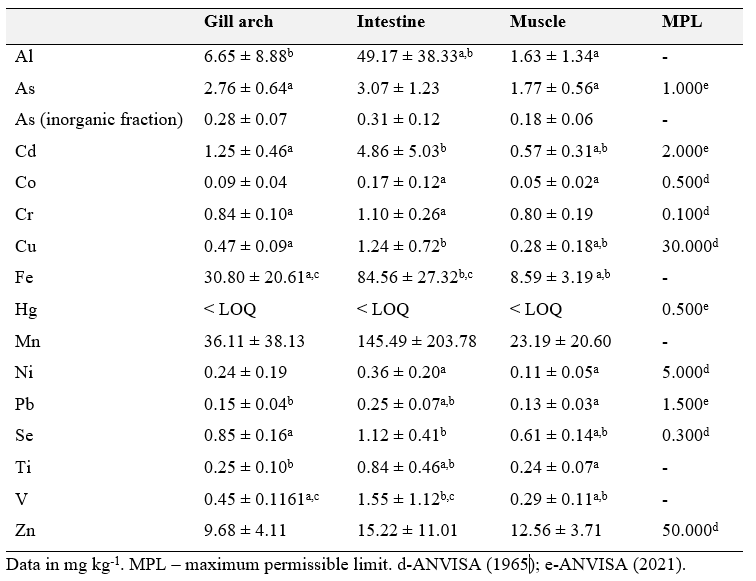
Al concentrations were lower in muscle, increasing in the gill arch and reaching the highest values in the intestines, significantly higher compared to muscle and gill arch (p = 0.0098 and p = 0.0157). For As, the highest concentration was observed in the intestine, followed by the gill arch and muscle, although only the gill arch values were significantly higher (p = 0.0087). This behavior was also similar for the inorganic fraction of element. Cd, despite significantly increased from the muscle to the gill arch (p = 0.0063), and from the muscle to the intestine (p = 0.0016), above the Brazilian guidelines of 2.0 mg kg-1 ww-1 (ANVISA, 2013), while intestine values reached up to 4.86 ± 5.03 mg kg-1 ww-1. The highest Co value was detected in the intestine, dropping to about half in the gill arch and again dropping to half in muscle. Cr concentrations were significantly higher in the intestine compared to the gill arch (p = 0.0086). For Cu, concentrations increased significantly between the muscle, 2gill arch and intestine, with the highest values observed in the latter. Cu concentrations in muscle were significantly higher than in the other tissues. For Fe, the highest value was detected in the intestine, significantly decreasing for the gill arch (p = 0.0023) and muscle tissue (p = 0.0008). All Hg values observed were below the method limit of quantification (LOQ). Mn values were higher in the intestines, although not statistically different from the other investigated tissues. No Brazilian standards for the maximum allowable concentration for Mn, Fe and Al are available for molluscs, and a maximum allowable concentration for Al is not established for aquatic organisms. Ni was significantly higher in the intestine when compared to muscle (p = 0.0086). Pb values were similar for muscle and gill arch, increasing significantly in the intestine (p = 0.0016 and p = 0033). Ti values behaved similarly, with a significant increase from muscle to gill arch (p = 0.0117) and muscle to the intestine (p = 0.0073). V was high in the intestine, decreasing in the gill arch and muscle, all significantly different (p = 0.0008). Zn, unlike the other metals and metalloids, was lowest in the gill arch, and exhibited similar values in the muscle and intestine.
The Tolerable weekly intake (PTWI) of scallop samples compared to the recommended PTWI and maximum permissible limit (MPL) for each element according to different regulating agencies worldwide are displayed in Table 3. The weekly intake was calculated summing the elemental concentrations for each analyzed tissue, as the animals are ingested whole.
Table 3. Estimate weekly intake (EWI) of scallop samples compared to the recommended PTWI and maximum permissible limit (MPL) for each element according to different regulating agencies worldwide.
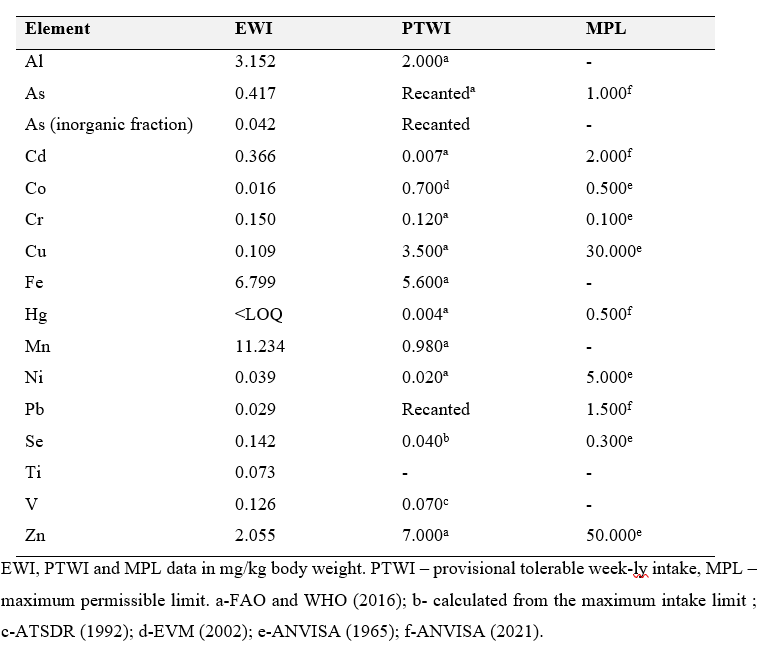
Al, Cd, Cr, Fe, Mn, Ni, Se and V were above the recommended PTWI, and cobalt, copper and zinc were below the recommended PTWI, while Pb and As were below MPL The THQ and TCR values calculated for the scallop samples analyzed herein are displayed in Table 4.
Table 4. THQ (target risk quotient) and TCR (target cancer risk) calculated for the different elements evaluated in the scallop samples analyzed herein.
DISCUSSION
Although there is no evidence about the causes of the mortality outbreaks that have been occurring in the study region, especially the role of metal and metalloid levels, analyzing them may allow conclusions to be drawn about the subject. In general, scallops accumulate higher metal and metalloid concentrations in the intestine, digestive gland and the gills, as reported in the Peruvian scallop Agropecten purpuratus (Loaiza et al., 2020), Farrer scallop (Chlamys farreri), Noble scallop (Chlamys nobilis), Atlantic scallop (Argopecten irradians), and Yesso scallop (Patinopecten yessonensis) (Lin et al., 2021), corroborating the highest levels observed herein in these organs.
Moderate negative correlations were observed between scallop length and Al (Rho = -0.800) and Ti Rho = 0.71) in the intestine, probably indicating a dilution effect and consequent metal detoxification, where increasing scallop lengths lead to decreased Al and Ti concentrations, as both relationships were negative (Hauser-Davis et al., 2021b). Moderate positive correlations for the intestine comprised Cd (Rho = -0.80) and Ni. (Rho = 0.71), indicating potential bioaccumulation processes in place with increasing scallop age. Systemic transport was noted for Co between muscle and gill arch (0.838), Mn between muscle and intestine (-0.738) and Pb between muscle and intestine (-0.810).
Several mollusc mortality events have occurred in Rio de Janeiro and eventually ceased without the identification of their causative agents (Landuci et al., 2021), indicating the need for continuous environmental monitoring and diagnoses capable of identifying the causes and suggesting treatment and better strategies channels to develop rapid responses, remediation, and preventive measures concerning the mollusc productive chain (Paladini et al., 2017).
Ilha Grande Bay, the sampling site evaluated herein, is considered a biodiversity hotspot, comprising many protected areas. However, a commercial port, two terminals (crude oil and minerals), shipyards and a nuclear energy complex are located in the area (Landuci et al., 2020), generating considerable anthropogenic impacts (Lino et al., 2016) as observed by Vannuci-Silva et al. (2017) who found higher concentrations of metals in the area close to these enterprises when compared to other sites spread over Ilha Grande bay. Even so, Zn, Fe, Cu, Mn, Cr, detected in the lion’s paw tissues were lower than the values recorded by Lino et al. (2016) in previous studies at the tip of Biscay, also in Ilha Grande Bay. Cd in muscle was, in fact, up to 1.9 x higher than that average level reported by the aforementioned authors, of 0.3 ug g-1 w.w (1.2 ug g-1 d.w).
Concerning sampling location, Vannuci-Silva et al. (2017) verified differences between metal concentrations during winter and summer campaigns when assessing metals in marine waters surrounding Ilha Grande Bay. The highest Cd concentrations were reported as 0.03 μg L-1 at Bananal, the same sampling area as the present study. Maximum levels of Cu (0.37 μg L−1); Pb (0.08 μg L−1); Co (0.15 μg L−1), Ni (0.27 μg L−1) were detected at Náutico (Figure 1), distant 7.4 miles from Bananal and near the city of Angra dos Reis, with many surrounding contamination sources, such as marinas, waste disposal sites, domestic sewage inputs, intense vessel traffic and river discharges.
Despite being located far from any contamination sources, Bananal is still highly affected by the winds that enter the bay (Vannuci-Silva et al., 2017), and can also receive considerable amounts of contaminants from others areas, such as Sepetiba Bay, which displays historically high Cd contamination levels(Galvão et al., 2010). In this regard, Guerra and Soares (2009), reported flows generated by meteorological process as low frequency oscillations when studying the advection of suspended particulate matter (SPM) from Sepetiba Bay through Ilha Grande Bay, supporting the sporadic occurrence of conditions that favor the introduction of particles originated from Sepetiba Bay to Ilha Grande Bay.
It is also necessary to consider that environmental characteristics such as seasonality are defining factors concerning metal and metalloid bioavailability in southeastern Brazil (Lino et al., 2016) and should, therefore, be considered in monitoring programs, resulting in metal level variations in cultivated areas (Knopf et al., 2020). Agriculture and aquaculture activities (Góngora-Gómez et al., 2018), the relative proximity to the pollutant input point , the position of the study organism in the water column (Barchiesi et al., 2020) and biological variables such as size, sex, or changes in tissue composition and reproductive cycle should also be considered, as seasonal fluctuations have been largely correlated with seasonal changes in mollusc weight during the development of gonadal tissues (Bustamante and Miramand, 2005).
Table 5 reports on metal accumulation levels in different scallops species and locations worldwide where scallops are important component of diet and aquaculture.
Table 5. Metal accumulation levels for Cd, Cu, Zn, Ni, Pb, Cr, Fe and Mn, and between scallop species and different locations worldwide. All data are expressed as μg. g-1w.w. FAAS – Flame Atomic Absorption Spectrometry, ICP-MS – Inductively Coupled Plasma Mass Spectrometry, ICP-OES – Inductively Coupled Optical Emission Spectrometry, ATFS – Atomic Fluorescence Spectrometry.
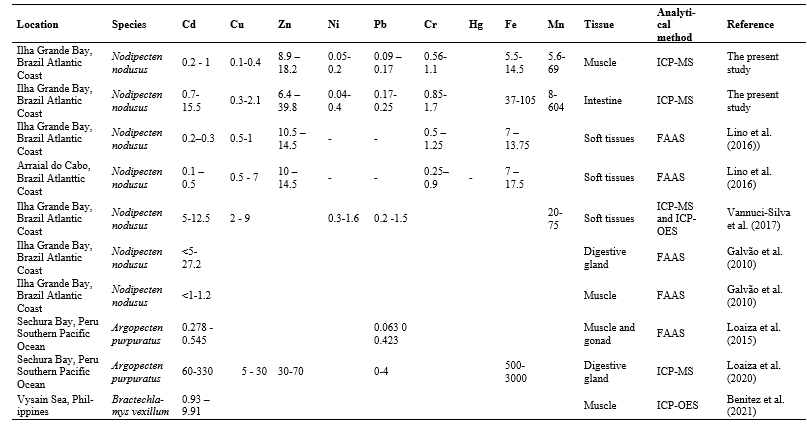
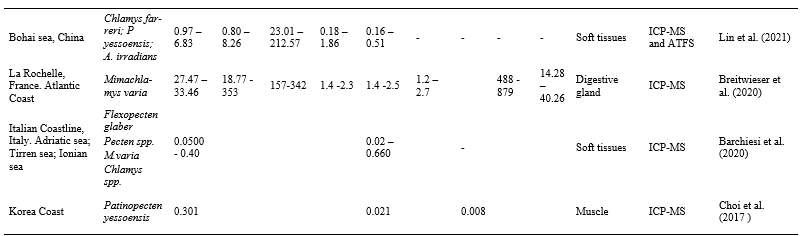
The Cd values detected in intestines in our study were similar to the values observed in Ilha Grande Bay by Vannuci-Silva et al. (2017) in soft tissues, but lower than those observed by (Galvão et al., 2010) in digestive glands. Concerning muscle, detected concentrations were similar to those observed by Galvão et al. (2010) in the same tissue and were below the 1 mg.kg-1 wet weight – European Union standard and 2.g.kg-1 Brazilian standard (Comissing Regulation, 2006;ANVISA, 2021). A. purpuratus scallops in Peru, presented Cd concentrations in muscle and gonads similar to those observed herein, but between 20-85-fold times higher in digestive glands (Loaiza et al., 2020) than observed herein for intestines. The concentration of the same element in Bractechlamys vexillum molluscs from the Philippines (Benitez et al. 2021), were 4-9-fold higher than those observed in the same tissues in N. nodusus. The values observed in soft tissues of C. farreri, P yessoensis and A. irradians in China were similar (Lin et al., 2021), while values in Flexopecten glaber, Pecten spp., M. varia and Chlamys spp. from Italy (Barchiesi et al., 2020) were lower than in the intestines of N. nodusus in our study. Cadmium in the digestive glands of M. varia from France (Breitwieser et al., 2020) were up to 40-fold higher than those observed in N. nodusus intestines. The Cd values in P. yessoensis muscle observed by Choi et al. (2017 ) in Korea were similar to the mean of the values observed herein. In general, digestive gland values were quite high compared to the other evaluated tissues. Soft tissue concentrations were similar, in general, to those observed in N. nodusus intestines and in the muscle tissues of different species. Cd is commonly detected in aquatic and terrestrial environments, released from natural sources (leaching of Cd-rich soils or volcanic activities), as well as from anthropogenic activities (production of plastics stabilizers and nickel-cadmium batteries, mining, electroplating, smelting, production of pigments). The presence in the environment of this heavy metal has been constantly increasing because of its large employment in several industrial and agricultural activities. Cadmium does not have any biological role and, since it cannot be degraded by living organisms, it is irreversibly accumulated into cells, interacting with cellular components and molecular targets. Cadmium is one of the most studied stress inductors and a potent modulator of several processes such as apoptosis, autophagy, reactive oxygen species, protein kinase and phosphatase, mitochondrial function, metallothioneins, and heat-shock proteins (Chiarelli et al., 2019).
Copper concentrations observed by Lino et al. (2016) in the same region, were similar in soft tissues to those observed in our study. The scallops from Arraial do Cabo, however, presented values 3-fold higher, although still lower than in other locations. Vannuci-Silva et al. (2017) reported values about 4-fold times higher for the same element in the soft tissues of scallops collected at Ilha Grande, similar to those detected in China by Lin et al. (2021) in the same tissues. In the digestive glands, Loaiza et al. (2015) observed values up to 15-fold higher, while Breitwieser et al. (2020) reported values between 9- and 170-fold higher for the species M. varia, compared to the intestines of N nodusus reported herein. Cu concentrations in the muscle of the samples collected in the present study were 1.77 ± 0.56 mg kg-1 w.w., there is no longer specific legislation for the limits of Cu in aquatic animals in Brazil, so the revoked legislation that established generic values for various foods was used as comparison (de Oliveira et al., 2022), and even so the values were below (30 mg kg-1 w.w.). High Cu concentrations in estuarine waters were related to be closer to areas under the influence of sewage disposal and vessels using copper-based antifouling paints (Lino et al., 2016). Copper is an essential trace element for some biological functions in macroalgal physiology. However, at high concentrations, copper can be toxic to macroalgae and to a wide range of other marine organisms (Leal et al., 2018).
The Zn values reported by Lino et al. (2016), both at Arraial do Cabo and Ilha Grande Bay, were similar to those observed in the muscle in the present study. On the other hand, in China, the soft tissues of the analyzed scallops were 5-fold higher than those found in intestines. In Peru and France. Zn in digestive glands were, respectively, 2- and 9-fold higher than the highest values observed in this study. The samples collected herein contained 12.56 ± 3.71, there is no longer specific legislation for the limits of Zn in aquatic animals in Brazil, so the revoked legislation that establishes generic values for various foods was used as comparison (de Oliveira et al., 2022), and even so the values were below (50 mg kg-1 w.w.)Zinc is a ubiquitous trace metal in the biosphere and geosphere, which is an essential micronutrient to organisms, but may be toxic in high concentrations. It is widely used in alloys, pesticides, electroplating and commonly associated to mining impacts due the economic importance of its ores. Bivalves in contaminated areas readily Zinc into both their soft tissues (predominately during feeding) and shells. In sufficient concentration, such metal contamination is toxic and therefore can significantly elevate mortality and impair growth rate. Incorporation of metals into mollusc shells occurs since elements such as Pb and Zn follow the same intracellular pathway as Ca during the biomineralization process (Stewart et al., 2021). Even though Zinc can compromise the growth of scallops, the redistribution of Zn between each subcellular component demonstrates that there is an effective regulatory mechanism in these animals (Pan and Wang, 2008).
Ni concentrations reported for soft tissues by Vannuci-Silva et al. (2017) at Ilha Grande Bay and in China (Lin et al., 2021) were about 4-fold higher than the values in intestine in our study. While values in scallops from France (Breitwieser et al., 2020) were 6-fold higher. Nickel is a non-biodegradable toxic heavy metal ion present in wastewater. In our study, a value of 0.11 ± 0.05 mg kg-1 w.w were detected in muscle, there is no longer specific legislation for the limits of Ni in aquatic animals in Brazil, so the revoked legislation that established generic values for various foods was used as comparison (de Oliveira et al., 2022), and even so the values were below (5 mg kg-1 w.w). The main source of nickel pollution in the water derives from a number of industrial production processes such as battery manufacturing, production of some alloys, zinc base casting, printing, electroplating and silver refineries (Yunus et al., 2020). Ni is a metal of high environmental relevance that has been shown to exert long-term toxic effects to aquatic biota such as microorganisms, invertebrates, particularly bivalves and fish. Ni was suspected to cause depletion of the antioxidant enzyme system and therefore it should be considered a pro-oxidant agent (Banni et al., 2014).
Pb exhibited a similar trend to Ni, with values detected in Peru for muscle (Loaiza et al., 2015) about 3-fold higher than in the same tissue as N. nodusus. In Italy (Barchiesi et al., 2020) values were similar for soft tissue. Our samples contained lower values (0.13 ± 0.03 mg kg-1 w.w) than the maximum limit values of Brazilian and European legislation (1.5 mg.kg-1) (Comissing Regulation, 2006;ANVISA, 2013) Marine contamination by Lead (Pb) is mainly due to releases from anthropogenic activities and the atmosphere constitutes the principal transport vector towards the Oceans. Pb is bioaccumulated by living organisms and more particularly by marine invertebrates. Pectinids are well known to accumulate high Pb levels in their tissues, and the kidneys and the digestive gland appeared to play an important role in the uptake and retention of this metal (Metian et al., 2009).
Cr in scallops in Ilha Grande Bay analyzed by (Lino et al., 2016) presented values similar to those observed in our study, whereas the values registered in France in digestive glands were almost 2-fold higher (Breitwieser et al., 2020) to those recorded by us in the intestines. The values registered in the muscles (0.80 ± 0.19 mg kg-1 w.w) were higher than those observed as limits in the Brazilian legislation (0.5 mg.kg-1) (ANVISA, 2021).Chromium pollution in aquatic environment is a major issue of concern as it is posing adverse impacts on human health and society directly or indirectly. This element enters the aquatic system through effluents from industries like textiles, tanneries, mining, electroplating, dyeing, printing, photographic printing, pharmaceuticals, stainless steel manufacturing and rubber manufacturing industries (Bakshi and Panigrahi, 2018). Growth reduction has been reported at EC50 concentrations of 0.51mg L-1 in scallop Argopecten ventricosus (Sobrino-Figueroa et al., 2007).
The Fe values in the muscle of N. nodusus detected in our study were similar to those observed by Lino et al. (2016) in the soft tissues of scallops of the same species at different sampling sites, and also lower than those observed in the intestine in our study. The values observed in the digestive glands by Loaiza et al. (2020), however, were approximately 30-fold times higher than the highest values recorded in our study. In France, for the same tissue, Breitwieser et al. (2020) recorded values approximately 8-fold higher. The coastal zone is influenced by Fe inputs from river waters, which may be transported a long distance. Rainwater is also a significant source of iron in surface waters, especially through rivers collecting drainage water and entering coastal waters. In recent years, as the economy has developed, anthropogenic sources such as industrial wastewater, sewage, agriculture irrigation, and other activities influence more and more the biogeochemical cycling of the dissolved Fe in coastal areas. Fe, although essential for growth and cellular functioning many biochemical reactions. May affect bivalve mollusc survival at high concentrations (González et al., 2010).
Similar Mn values were recorded in the muscle tissue of N. nodusus from Ilha Grande Bay (Vannuci-Silva et al., 2017) and to the tissue of the digestive glands in France (Breitwieser et al., 2020) compared to our study. In our study, the values obtained for the intestine were between 8 and 15 times higher. Mn is a biologically essential trace metal, a major global commodity and an emerging marine contaminant for which ecotoxicological data are still inadequate. Background concentrations range from 0.03 – 10 μg L-1 in open seawater and 0.2–26 μg L-1 in coastal waters, depending on local geology. On other hand, higher concentrations (> 30 mg L-1) occur under suboxic conditions and as a result of anthropogenic contamination from sources including runoff and leaching from metal ore extraction and processing sites, tailings dam seepage and collapse, submarine tailings disposal, channel dredging, land reclamation, urban and industrial effluents, agricultural runoff, erosion, and atmospheric deposition (Summer et al., 2019). Manganese is an essential element for plants and animals, as an integral part of many enzymes and is is among the least toxic of all metals to animals (Milatovic and Gupta, 2012).
Regarding Se benefits obtained from the ingestion of the analyzed scallops, our estimated weekly intake was calculated as 0.142 mg kg-1 bw-1, higher that the weekly Recommended Dietary Allowances for adults of 0.0385 mg kg-1 bw-1 by the Institute of Medicine (2000) and NIH (2021), and the PTWI of 0.040 mg kg-1 bw-1 established by the (WHO and FAO, 2004) for human consumption, but still lower than the maximum permissible limit of 0.300 mg kg-1 established by(ANVISA, 1965). As Hg values were lower than the LOQ, it seems that no harm from this element is noted regarding the consumption of scallops from the sampled location. In addition, Se values are likely to be protective of any low concentrations below the LOQ present in these molluscs (Ralston et al., 2016). Lino et al. (2016), investigating the bioaccumulation of metals in mussels and scallops grown in different areas of the state of Rio de Janeiro, found that Cd and Mn are more efficiently accumulated by scallops and that the consumption of the two species is not considered safe, due to high Cu and Cr concentrations above the established limits by the Brazilian Health Surveillance Agency (ANVISA), similarly to our findings.
All THQ values were below 1, indicating that it is unlikely that consumers will experience obvious adverse effects from the consumption of scallops from the sampling location (Wang et al., 2005;Taweel et al., 2013). However, since the range for acceptable risk of developing cancer is reported as 10-6 to 10-4 (US-EPA, 1991;Shaheen et al., 2016), concerns may be noted with regard to Cr, Ni and Pb, as Cr is classified as an IARC group 1 (carcinogenic to humans), Ni is classified as an IARC group 2B (suspected human carcinogen and Pb is classified as an IARC group 2 A (probable human carcinogen) (IARC, 2021).
Modern aquatic food consumers generally support sustainable production. Linking ecosystem services to reputable certification schemes, in conjunction with the development of jurisdictional management and regulatory frameworks, could create a pathway to the intentional delivery of benefits by operators while deterring the occurrence of unaddressed negative impacts (Alleway et al., 2018). Furthermore, incorporating valuations into existing seafood certification schemes (e. g., Best Aquaculture Practices, the Aquaculture Stewardship Council (ASC), Global GAAP, the Monterey Bay Seafood Watch program) might facilitate greater market recognition of these services. Also, health certifications restrict access to credits, in in this scenario monitoring programs could contribute to solve these issues (Alleway et al., 2018;Landuci et al., 2021). However, for this to be successful, bivalve aquaculture operations must, at a minimum, adhere to national and local laws. For example ASC Bivalve Standards may develop sustainability requirements beyond those required by law, but the baseline requirement for any aquaculture operation must be compliance with the legal obligations of the producing country (ASC, 2019).
The findings reported herein indicate the importance of monitoring the presence of metals and metalloids lion’s paw scallops destined for human consumption in southeastern Brazil. Brazilian health authorities should conduct sanitary quality management of marine bivalve molluscs in a program that considers local seasonality variations, mollusc reproductive efforts and the logistics for the destination of residential and industrial sewage, in line with a sustainable planning seeking to avoid the pollution of estuaries and coastal strips.
ACKNOWLEDGEMENTS
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ. The authors appreciate the support of the Instituto de Eco Desenvolvimento da Baía da Ilha Grande – IED-BIG and Dr. Felipe Aníbal, from Laboratory of Epidemiology and Molecular Systematics at the Oswaldo Cruz Institute.
REFERENCES
ALLEWAY, H. K., GILLIES, C. L., BISHOP, M. J., GENTRY, R. R., THEUERKAUF, S. J. & JONES, R. 2018. The Ecosystem Services of Marine Aquaculture: Valuing Benefits to People and Nature. BioScience, 69, 59-68.
ANVISA. 1965. Modifica o Decreto nº 50.040, de 24 de janeiro de 1961, referente a normas reguladoras do emprêgo de aditivos para alimentos, alterado pelo Decreto nº 691, de 13 de março de 1962. In: ANVISA (ed.) Decreto n° 55871,. Brasilia, Brasil: Diario Oficial da União.
ANVISA. 2013. Resolução RDC n°42 de 29 de Agosto de 2013. In: SAÚDE, M. D. (ed.). Brasilia, Brasil: Imprensa Oficial – Diario Oficial da União.
ANVISA. 2021. In N° 88, de 26 de Março de 2021, estabelece os limites máximos tolerados (LMT) de contaminantes em alimentos. In: AGENCY, B. H. R. (ed.). Brasilia, Brazil: Diario Oficial da União, Imprensa Oficial.
ARZUL, I., CORBEIL, S., MORGA, B. & RENAULT, T. 2017. Viruses infecting marine molluscs. Journal of Invertebrate Pathology, 147, 118-135.
ASC. 2019. ASC Bivalve Standard. v.1.1. Utrecht, Netherlands: ASC.
ATSDR. 1992. Toxicological Profile for Vanadium. In: DEPARTMENT OF HEALTH AND HUMAN SERVICES (ed.). Atlanta, USA: Public Health Service.
BAKSHI, A. & PANIGRAHI, A. K. 2018. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicology reports, 5, 440-447.
BANNI, M., HAJER, A., SFORZINI, S., OLIVERI, C., BOUSSETTA, H. & VIARENGO, A. 2014. Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 160, 23-29.
BARCHIESI, F., BRANCIARI, R., LATINI, M., ROILA, R., LEDIANI, G., FILIPPINI, G., SCORTICHINI, G., PIERSANTI, A., ROCCHEGIANI, E. & RANUCCI, D. 2020. Heavy Metals Contamination in Shellfish: Benefit-Risk Evaluation in Central Italy. 9, 1720.
BENITEZ, K. C. D., CAMBIA, F. D., BANICOD, R. J. S., PERELONIA, K. B. S., TADIFA, G. C., TANYAG, B. E., RIVERA, A. T. F. & MONTOJO, U. M. 2021. Concentrations, seasonality, and risk assessment of cadmium in scallop, Bractechlamys vexillum (Reeve 1853) in the Visayan Sea, Philippines. Food Control, 126, 108021.
BREITWIESER, M., BARBARIN, M., PLUMEJEAUD-PERREAU, C., DUBILLOT, E., GUYOT, T., HUET, V., CHURLAUD, C., COULOMBIER, T., BRENON, I., FICHET, D., IMBERT, N. & THOMAS, H. 2020. Biomonitoring of Mimachlamys varia transplanted to areas impacted by human activities (La Rochelle Marina, France). Chemosphere, 243, 125199.
BRYMAN, A. & CRAMER, D. 2011. Quantitative data analysis with IBM SPSS 17, 18 and 19, Routledge, 408 pp.
BUSTAMANTE, P. & MIRAMAND, P. 2005. Evaluation of the variegated scallop Chlamys varia as a biomonitor of temporal trends of Cd, Cu, and Zn in the field. Environmental Pollution, 138, 109-120.
CAPILLO, G., SILVESTRO, S., SANFILIPPO, M., FIORINO, E., GIANGROSSO, G., FERRANTELLI, V., VAZZANA, I. & FAGGIO, C. 2018. Assessment of Electrolytes and Metals Profile of the Faro Lake (Capo Peloro Lagoon, Sicily, Italy) and Its Impact on Mytilus galloprovincialis. 15, 1800044.
CHIARELLI, R., MARTINO, C. & ROCCHERI, M. C. 2019. Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell stress & chaperones, 24, 675-687.
CHOI, W. S., YOON, M., JO, M. R., KWON, J. Y., KIM, J. H., LEE, H. J. & KIM, P. H. 2017 Heavy Metal Contents in Internal Organs and Tissues of Scallops Patinopecten yessoensis and Comb Pen Shell Atrina pectinata. Korean Journal of Fisheries and Aquatic Science, 50, 487-493.
COMISSING REGULATION, E. 2006. setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance. In: REGULATION, C. (ed.) 1881/2016. UE: Official Journal of the European Union.
DE OLIVEIRA, A. G. L., ROCHA, R. C. C., SAINT’PIERRE, T. D., HAUSER-DAVIS, R. A., MELLO-SILVA, C. C. & SANTOS, C. P. 2022. Elemental Contamination in Brown Mussels (Perna perna) Marketed in Southeastern Brazil. Biological Trace Element Research, 200, 402-412.
EVM. 2002. Review of Cobalt. In: MINERALS, T. E. G. O. V. A. (ed.). www.foodstandards.gov.uk.
FAO. 2020. The State of World Fisheries and Aquaculture 2020 – Sustainability in Action. The State of World Fisheries and Aquaculture. Rome, Italy.
FAO & WHO. 2018. Technical guidance for the development of the growing area aspects of Bivalve Mollusc Sanitation Programmes. In: WHO, F. A. (ed.) Food Safety and Quality Series. Rome: FAO and WHO.
GALVÃO, P., TORRES, J., MALM, O. & REBELO, M. 2010. Sudden cadmium increases in the digestive gland of scallop, Nodipecten nodosus L., farmed in the tropics. Bull Environ Contam Toxicol, 85, 463-466.
GALVÃO, P. M. A., REBELO, M. F., GUIMARÃES, J. R. D., TORRES, J. P. M. & MALM, O. 2009. Bioacumulação de metais em moluscos bivalves: Aspectos evolutivos e ecológicos a serem considerados para a biomonitoração de ambientes marinhos. Brazilian Journal of Aquatic Science and Technology, 13, 59-66.
GÓNGORA-GÓMEZ, A. M., DOMÍNGUEZ-OROZCO, A. L., VILLANUEVA-FONSECA, B. P., MUÑOZ-SEVILLA, N. P. & GARCÍA-ULLOA, M. 2018. SEASONAL LEVELS OF HEAVY METALS IN SOFT TISSUE AND MUSCLE OF THE PEN SHELL Atrina maura (SOWERBY, 1835) (BIVALVIA: PINNIDAE) FROM A FARM IN THE SOUTHEASTERN COAST OF THE GULF OF CALIFORNIA, MEXICO. Revista Internacional de Contaminación Ambiental, 34, 57-68.
GONZÁLEZ, P. M., ABELE, D. & PUNTARULO, S. 2010. Exposure to excess dissolved iron in vivo affects oxidative status in the bivalve Mya arenaria. Comp Biochem Physiol C Toxicol Pharmacol, 152, 167-174.
GUERRA, J. V. & SOARES, F. L. M. 2009. Circulation and flux of suspended particulate matter in Ilha Grande Bay, SE Brazil. Journal of Coastal Research, 1350-1354.
HAUSER-DAVIS, R. A., LAVRADAS, R. T., MONTEIRO, F., ROCHA, R. C. C., BASTOS, F. F., ARAÚJO, G. F., SALES JÚNIOR, S. F., BORDON, I. C., CORREIA, F. V., SAGGIORO, E. M., SAINT’PIERRE, T. D. & GODOY, J. M. 2021a. Biochemical metal accumulation effects and metalloprotein metal detoxification in environmentally exposed tropical Perna perna mussels. Ecotoxicology and Environmental Safety, 208, 111589.
HAUSER-DAVIS, R. A., SILVA-JUNIOR, D. R., LINDE-ARIAS, A. R. & VIANNA, M. 2021b. Cytosolic and Metallothionein-Bound Hepatic Metals and Detoxification in a Sentinel Teleost, Dules auriga, from Southern Rio de Janeiro, Brazil. Biological Trace Element Research, 199, 744-752.
IARC. 2021. Agents Classified by the IARC Monographs, Volumes 1–129. In: CANCER, I. A. F. R. O. (ed.) IARC MONOGRAPHS ON THE IDENTIFICATION OF CARCINOGENIC HAZARDS TO HUMANS. https://monographs.iarc.who.int/agents-classified-by-the-iarc/: World Health Organization.
IBGE. 2021. Rio de Janeiro [Online]. [Accessed 20/05 2021].
INMETRO. 2020. Orientação Sobre Validação de Métodos Analíticos (DOQ-CGCRE-008). In: ACREDITAÇÃO, C. G. D. (ed.). Instituto Nacional de Metrologia, Normalização e Qualidade Industrial Inmetro.
INSTITUTE OF MEDICINE. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, The National Academies Press, Washington, DC, 528 pp.
KLUGER, L. C., TAYLOR, M. H., WOLFF, M., STOTZ, W. & MENDO, J. 2019. From an open-access fishery to a regulated aquaculture business: the case of the most important Latin American bay scallop (Argopecten purpuratus). Reviews in Aquaculture, 11, 187-203.
KNOPF, B., FLIEDNER, A., RADERMACHER, G., RÜDEL, H., PAULUS, M., PIRNTKE, U. & KOSCHORRECK, J. 2020. Seasonal variability in metal and metalloid burdens of mussels: using data from the German Environmental Specimen Bank to evaluate implications for long-term mussel monitoring programs. Environmental Sciences Europe, 32, 7.
LANDUCI, F. S., BEZ, M. F., RITTER, P. D., COSTA, S., SILVESTRI, F., ZANETTE, G. B., CASTELAR, B. & SANTOS COSTA, P. M. 2021. Mariculture in a densely urbanized portion of the Brazilian coast: Current diagnosis and directions for sustainable development. Ocean & Coastal Management, 213, 105889.
LANDUCI, F. S., RODRIGUES, D. F., FERNANDES, A. M., SCOTT, P. C. & POERSCH, L. H. D. S. 2020. Geographic Information System as an instrument to determine suitable areas and identify suitable zones to the development of emerging marine finfish farming in Brazil. Aquaculture Research, 51, 3305-3322.
LEAL, D. A. G. & FRANCO, R. M. B. 2008. Bivalve Molluscs destined to human consumption as vectors of pathogenic protozoa:detection methodologies and control rules. Revista Panamericana de Infectologia, Rev. Pan. Infectol, 48-57.
LEAL, P. P., HURD, C. L., SANDER, S. G., ARMSTRONG, E., FERNÁNDEZ, P. A., SUHRHOFF, T. J. & ROLEDA, M. Y. 2018. Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Scientific Reports, 8, 14763.
LÉVESQUE, B., BARTHE, C., DIXON, B. R., PARRINGTON, L. J., MARTIN, D., DOIDGE, B., PROULX, J. F. & MURPHY, D. 2010. Microbiological quality of blue mussels (Mytilus edulis) in Nunavik, Quebec: a pilot study. Can J Microbiol, 56, 968-977.
LIN, Y., LU, J. & WU, J. 2021. Heavy metals pollution and health risk assessment in farmed scallops: Low level of Cd in coastal water could lead to high risk of seafood. Ecotoxicology and Environmental Safety, 208, 111768.
LINO, A. S., GALVÃO, P. M. A., LONGO, R. T. L., AZEVEDO-SILVA, C. E., DORNELES, P. R., TORRES, J. P. M. & MALM, O. 2016. Metal bioaccumulation in consumed marine bivalves in Southeast Brazilian coast. Journal of Trace Elements in Medicine and Biology, 34, 50-55.
LOAIZA, I., HURTADO, D., MIGLIO, M., ORREGO, H. & MENDO, J. 2015. Tissue-specific Cd and Pb accumulation in Peruvian scallop (Argopecten purpuratus) transplanted to a suspended and bottom culture at Sechura Bay, Peru. Marine Pollution Bulletin, 91, 429-440.
LOAIZA, I., PILLET, M., DE BOECK, G. & DE TROCH, M. 2020. Peruvian scallop Argopecten purpuratus: From a key aquaculture species to a promising biondicator species. Chemosphere, 239, 124767.
METIAN, M., WARNAU, M., OBERHÄNSLI, F. & BUSTAMANTE, P. 2009. Delineation of Pb contamination pathways in two Pectinidae: the variegated scallop Chlamys varia and the king scallop Pecten maximus. Sci Total Environ, 407, 3503-3509.
MILATOVIC, D. & GUPTA, R. C. 2012. Chapter 38 – Manganese. In: GUPTA, R. C. (ed.) Veterinary Toxicology (Second Edition). Academic Press, Boston, pp. 527-536.
NIH. 2021. Selenium [Online]. [Accessed 10/24/2021 2021].
ORY, P., HAMANI, V., BODET, P. E., MURILLO, L. & GRABER, M. 2021. The variegated scallop, Mimachlamys varia, undergoes alterations in several of its metabolic pathways under short-term zinc exposure. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 37, 100779.
PALADINI, G., LONGSHAWN, M., GUSTINELLI, A. & SHINN, A. 2017. Parasitic Diseases in Aquaculture: Their Biology, Diagnosis and Control. In: AUSTIN, B. & NEWAJ‐FYZUL, A. (eds.) Diagnosis and Control of Diseases of Fish and Shellfish. pp. 37-107.
PAN, K. & WANG, W.-X. 2008. The subcellular fate of cadmium and zinc in the scallop Chlamys nobilis during waterborne and dietary metal exposure. Aquatic Toxicology, 90, 253-260.
PEREIRA, C. S., POSSA, C. A., VIANA, C. M. & RODRIGUES, D. P. 2007. Características de Vibrio parahaemolyticus isolados de mexilhões (Perna perna) comercializados em Niterói, Rio de Janeiro. Revista da Sociedade Brasileira de Medicina Tropical [online], 40, 56-59.
POTASMAN, I., PAZ, A. & ODEH, M. 2002. Infectious Outbreaks Associated with Bivalve Shellfish Consumption: A Worldwide Perspective. Clinical Infectious Diseases, 35, 921-928.
RALSTON, N. V. C., RALSTON, C. R. & RAYMOND, L. J. 2016. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biological trace element research, 171, 262-269.
ROMERO-MURILLO, P., ESPEJO, W., BARRA, R. & ORREGO, R. 2017. Embryo–larvae and juvenile toxicity of Pb and Cd in Northern Chilean scallop Argopecten purpuratus. Environmental Monitoring and Assessment, 190, 16.
SALLENAVE-NAMONT, C., POUCHUS, Y. F., ROBIOU DU PONT, T., LASSUS, P. & VERBIST, J. F. 2000. Toxigenic saprophytic fungi in marine shellfish farming areas. Mycopathologia, 149, 21-25.
SHAHEEN, N., IRFAN, N. M., KHAN, I. N., ISLAM, S., ISLAM, M. S. & AHMED, M. K. 2016. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere, 152, 431-438.
SOBRINO-FIGUEROA, A. S., CÁCERES-MARTÍNEZ, C., BOTELLO, A. V. & NUNEZ-NOGUEIRA, G. 2007. Effect of cadmium, chromium, lead and metal mixtures on survival and growth of juveniles of the scallop Argopecten ventricosus (Sowerby II, 1842). Journal of Environmental Science and Health, Part A, 42, 1443-1447.
SOUZA, D. A., ZANETTE, G. B., NEVES, M. H. C. B., SCHRAMM, M., PROENÇA, L. A. O. & OLIVEIRA, M. 2015. Cultivo de Moluscos Bivalves: Algas Nocivas e Bases para Programa de Monitoramento de Ficotoxinas em Fazenda de Maricultura de Arraial do Cabo, RJ. Boletim do Observatório Ambiental Alberto Ribeiro Lamego, 9, 119-139.
STEWART, B. D., JENKINS, S. R., BOIG, C., SINFIELD, C., KENNINGTON, K., BRAND, A. R., LART, W. & KRÖGER, R. 2021. Metal pollution as a potential threat to shell strength and survival in marine bivalves. Science of The Total Environment, 755, 143019.
SUMMER, K., REICHELT-BRUSHETT, A. & HOWE, P. 2019. Toxicity of manganese to various life stages of selected marine cnidarian species. Ecotoxicol Environ Saf, 167, 83-94.
TAWEEL, A., SHUHAIMI-OTHMAN, M. & AHMAD, A. K. 2013. Assessment of heavy metals in tilapia fish (Oreochromis niloticus) from the Langat River and Engineering Lake in Bangi, Malaysia, and evaluation of the health risk from tilapia consumption. Ecotoxicol Environ Saf, 93, 45-51.
US-EPA. 1989. Risk Assessment Guidance for Superfund, vol. I. Human Health Evaluation Manual (Part A), Interim Final. In: AGENCY, U. S. E. P. (ed.). Washington, USA: Environmental Protection Agency.
US-EPA. 1991. Guidelines for developmental toxicity risk assessment. In: AGENCY, U. S. E. P. (ed.). Washington, United States: Federal Register.
US-EPA. 2000. Risk Assessment and Fish Consumption Limits In: EPA, U. (ed.) Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories. Third Edition ed. Washington, USA: United States Environmental Protection Agency.
USP. 2013. Elemental Impurities – Procedures, 38–NF 33 second supplement. In: PHARMACOPEIA, U. S. (ed.). Maryland, USA: United States Pharmacopeia
VALENTI, W. C., BARROS, H. P., MORAES-VALENTI, P., BUENO, G. W. & CAVALLI, R. O. 2021. Aquaculture in Brazil: past, present and future. Aquaculture Reports, 19, 100611.
VANNUCI-SILVA, M., DE SOUZA, J. M., DE OLIVEIRA, F. F., DE ARAÚJO, M. A. G., FRANCIONI, E., EISMANN, C. E., KIANG, C. H., GOVONE, J. S., BELZUNCE-SEGARRA, M. J. & MENEGÁRIO, A. A. 2017. Bioavailability of Metals at a Southeastern Brazilian Coastal Area of High Environmental Concern Under Anthropic Influence: Evaluation Using Transplanted Bivalves (Nodipecten nodosus) and the DGT Technique. Water, Air, & Soil Pollution, 228, 222.
WANG, X., SATO, T., XING, B. & TAO, S. 2005. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Science of The Total Environment, 350, 28-37.
WHO & FAO. 2004. Vitamin and mineral requirements in human nutrition. Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998. In: WHO & FAO (eds.) 2nd ed ed. Geneva: World Health Organization.
WITTMAN, R. J. & FLICK, G. J. 1995. Microbial contamination of shellfish: prevalence, risk to human health, and control strategies. Annu Rev Public Health, 16, 123-140.
XU, L., WANG, Z., ZHAO, J., LIN, M. & XING, B. 2020. Accumulation of metal-based nanoparticles in marine bivalve mollusks from offshore aquaculture as detected by single particle ICP-MS. Environmental Pollution, 260, 114043.
YUNUS, K., ZURAIDAH, M. A. & JOHN, A. 2020. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofeminism and Climate Change, 1, 21-35.
1 Fundação Oswaldo Cruz, Centro de Desenvolvimento Tecnológico em Saúde – CDTS, Av. Brasil, 4365 – Manguinhos CEP 210401210, Rio de Janeiro-RJ/ Brasil. antonia.lucia@fiocruz.br
2 Laboratório de Epidemiologia e Sistemática Molecular, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Av. Brasil 4365 – Manguinhos, Rio de Janeiro CEP 21040-360, Brasil.
3 Fundação Instituto de Pesca do Rio de Janeiro – Diretoria de Pesquisa e Produção – Av. das Américas 31.501 – 23032051 – Rio de Janeiro, RJ – Brasil. Orcid: 0000-0001-7945-6796. Orcid: 0000-0001-7945-6796
4 Instituto de Eco Desenvolvimento da Baía de Ilha Grande – IED-BIG/Angra-RJ.
5 Departamento de Química, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, RJ, 22453-900, Brasil.
6 Laboratório de Avaliação e Promoção da Saúde Ambiental, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Av. Brasil, 4.365, Manguinhos, Rio de Janeiro, RJ, 21040-360, Brasil. Orcid: 0000-0002-9451-471X.

