REGISTRO DOI: 10.69849/revistaft/fa10202411300847
Kariny Rodrigues Tófoli,
Clara Madureira Siqueira Rodrigues,
Daniel Sossai Altoé,
Rodrigo Neves Ferreira,
Antônio Chambô Filho
ABSTRACT
Introduction: Endometrial stromal sarcomas (ESS) are rare mesenchymal tumors that predominantly affect perimenopausal women. These tumors are classified into endometrial stromal nodules, low-grade ESS, high-grade ESS, and undifferentiated sarcomas. This study reports a rare case of high-grade ESS in a 19-year-old woman treated at the Gynecology and Clinical Oncology Department of Santa Casa de Misericórdia Hospital in Vitória, Brazil. The patient presented with abnormal uterine bleeding, low hematimetric indices, abdominal pain, and a friable, cerebroform lesion with foul-smelling discharge on vaginal examination. Histopathological and immunohistochemical analyses confirmed the diagnosis of high-grade ESS. Surgical treatment was performed, and pathological evaluation indicated that the lesion originated from the endocervix. Adjuvant radiotherapy was recommended during the oncology consultation, but the patient lost follow-up and did not undergo this treatment. ESS diagnosis is challenging and often confused with other tumors, making differential diagnosis of abnormal uterine bleeding critical. This case highlights the importance of early diagnosis and appropriate treatment.
Keywords: High-grade endometrial stromal sarcoma; Young woman; Uterine cervix; Abnormal uterine bleeding; Lower genital tract pathology.
INTRODUCTION
Endometrial stromal sarcomas (ESS) are rare mesenchymal tumors originating from the uterus, comprising less than 2% of all uterine tumors (Eamudomkarn & Nuntasiri, 2018). Involvement of sites outside the uterine body, such as the uterine cervix, vagina, and extragenital organs, is even rarer (Abell & G, 1973; Hasiakos & D, 2007; Usha et al., 2014). ESS typically affects women in perimenopause, with few cases reported in younger patients (Akaév, 2021). Clinically, ESS presents with abnormal uterine bleeding or pelvic pain, though asymptomatic cases are also possible. Tumor prolapse through the uterine cervix can occur in rare instances (Usha et al., 2014).
According to the 2020 World Health Organization (WHO) classification, ESS is categorized into four types: endometrial stromal nodule, low-grade ESS, high-grade ESS, and undifferentiated uterine sarcoma. Among these, low-grade ESS is the most common, accounting for approximately 60% of cases (Azevedo et al., 2022; Eamudomkarn et al., 2021).
This article presents a rare case of high-grade ESS in a 19-year-old woman with endocervical involvement and abnormal uterine bleeding. Few cases in the literature associate this histological subtype with early age and the uncommon location of the uterine cervix. Thus, this case report is crucial to expand the understanding of this pathology and contribute valuable insights into this rare and under-reported pathology.
CASE REPORT
We describe the case of a 19-year-old woman diagnosed with high-grade ESS in the endocervical region, who was treated at the Gynecology and Clinical Oncology Department of Santa Casa de Misericórdia Hospital in Vitória, Brazil (HSCMV). The study was approved by the Research Ethics Committee (approval number 7.035.535).
The patient, Vitoria (ES) citizen, a G2P1(C1)A1, smoker and occasional user of alcohol and cannabis, had comorbidities including epilepsy, generalized anxiety disorder, and untreated depression. She presented at the Emergency department with a 6-month history of abnormal uterine bleeding, nausea, headache, asthenia, and unobserved tonic-clonic seizures.
Upon admission, the patient was pale, dehydrated, tachycardic, and normotensive, with severe anemia requiring blood transfusion. On speculum examination, a large, friable, cerebroform lesion was observed in the vaginal fornix, accompanied by foul-smelling discharge resembling “meat water.” The lesion obscured the uterine cervix, which was not visible. On vaginal examination, it was noted that the lesion protruded from the external cervical os. Thus, hospitalization was chosen for stabilization and further investigation of the condition.
A transvaginal doppler ultrasound showed a solid, hyperechoic lesion in the uterine cervix, extending into the vaginal fornix, measuring 71 x 58 mm, score doppler 4 (Figure A).
After improvement in hematimetric indices and clinical stabilization, excision of the polypoid lesion was performed via the vaginal route. A friable, irregular, and soft mass measuring approximately 7 cm was removed and sent for histopathological analysis.

Figure A – Endovaginal ultrasonography showing a solid and echogenic image projecting into the vaginal fornix and endocervix, measuring 71 x 58 mm. Source: Author (2022).
Histopathological examination revealed high-grade malignant features, and immunohistochemical analysis confirmed the diagnosis of high-grade ESS, which showed positivity for cyclin D1, CD10, and Ki-67 (Table 1).
Table 1 – Immunohistochemical findings of the lesion.
IMMUNOHISTOCHEMISTRY Pan-Cytokeratin (clone AE1AE3) Negative Carcinoembryonic Antigen (CEA, clone ii-7) Negative CD10 (CALLA, clone 56C6) Positive Cyclin D1 (clone EP12) Positive Ki-67 (MIB-1) Positive (60%)
Source: Author (2022).
For metastasis screening, computed tomography (CT) scans of the upper abdomen, pelvis, and chest were requested. The imaging revealed a thin-walled cystic structure in the hypogastric region, measuring 6.7 x 7 x 7 cm, likely of adnexal origin, with no evident enhancement, lymphadenopathy, or other abnormalities.
During an outpatient consultation, a new physical examination identified a lesion approximately 4 cm in size, centrally located on the uterine cervix, protruding from the external cervical os. The lesion was friable, soft, and bleeding. Rectal examination revealed no parametrial induration.
Given the patient’s young age, surgical management was chosen, and a hysterectomy with bilateral salpingectomy was performed. Intraoperatively, macroscopic alterations were observed in the left ovary, prompting oophorectomy. Histopathological examination of the surgical specimen showed a uterine cavity with a virtual endometrial cavity, an endometrial thickness of 0.3 cm, and a myometrial thickness of 1.3 cm. In the endocervix, a brown, elastic polyp measuring 2.5 x 2 x 1.6 cm was observed.
Microscopic analysis revealed a high mitotic activity area with hyperchromatic cells originating from the stroma, accompanied by intense vascularization, leading to a diagnosis of high-grade malignant neoplasm. Immunohistochemistry confirmed the diagnosis of high-grade endometrial stromal sarcoma. No angiolymphatic invasion was identified, and surgical margins and adnexa were free of neoplasia.
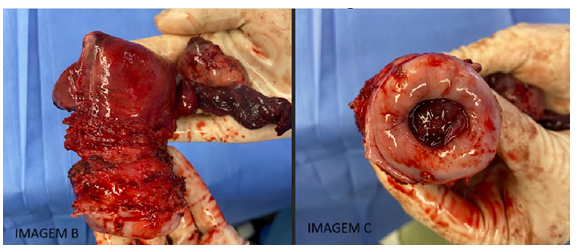
Figures B, C, D, and E below illustrate the anatomical specimens and slides highlighting the structural alterations.
Figures B and C: Figure B: Coronal view of the uterus, fallopian tubes, and left ovary. Displays friable, hyperemic tissue with an endometrial thickness of 0.3 cm and myometrial thickness of 1.3 cm. Figure C: Cervix with a brown lesion measuring 2.5 x 2 x 1.6 cm.
.
Source: Author (2022).
The patient was referred for adjuvant radiotherapy, but she lost follow-up, and despite efforts to contact her, treatment was not administered. She remains under clinical surveillance, with no evidence of disease recurrence to date.
Figure D: The microscopic examination reveals a tumor with high mitotic activity, featuring hyperchromatic cells in a hypervascularized stroma (H&E staining, 200x magnification).
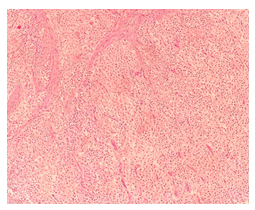
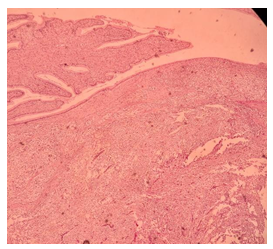
Figure E: Presence of cellular proliferation with high mitotic activity in the endocervical stroma, with adjacent normal endocervical epithelium. (H&E staining, 100x magnification).
The patient was referred to the clinical oncology team, and radiotherapy was recommended. However, the patient lost follow-up with oncology and did not undergo the proposed treatment, missing the window for adjuvant therapy. Currently, she is under joint follow-up with the clinical and surgical oncology teams, with no evidence of disease recurrence.
DISCUSSION
Endometrial Stromal Sarcomas (SEE) are rare tumors, accounting for less than 10% of uterine sarcomas and less than 1% of malignant uterine neoplasms. They belong to a subset of uterine mesenchymal neoplasms and are classified as homologous sarcomas, meaning they contain only elements that are naturally present in the uterus (Huang, 2019; Akaev et al., 2021).
Risk factors include estrogen use, tamoxifen therapy, chronic anovulation syndrome, and pelvic radiation. Approximately 50% of SEE cases occur during the perimenopausal period, and only a few cases have been reported in young women (Akaev, 2021).
Clinically, SEE presents with symptoms such as abnormal uterine bleeding, pelvic pain, abdominal distension, and in 25% of cases, it may be asymptomatic. The most common site of involvement is the uterus; however, cases have also been reported in extra-uterine locations such as the omentum, ovary, vulva, and vagina (Usha; M, 2014). Rarely, endocervical involvement by this pathology has been reported. In their study, Usha et al. describe the case of a young woman with low-grade SEE in an endocervical polyp. Another similar case of low-grade SEE is reported by Boardman et al. in a woman with breast cancer undergoing hormonal therapy. Both cases refer to a low-grade histological subtype. In this report, we describe a case of high-grade endometrial stromal sarcoma in a young 19-year-old patient, located in the endocervical region, presenting with abnormal uterine bleeding as the main complaint.
Endometrial stromal sarcomas (SEE) are rare tumors, and their diagnosis is often made through histopathological analysis of the surgical specimen, as biopsy alone is generally insufficient to distinguish vascular changes, infiltration, and invasion, which are necessary for classifying the tumor into benign or malignant subtypes (Brooks et al., 2004; Rauh-Hain, Del Carmen, 2013; Capozzi et al., 2020). In this case, as the patient had a lesion in the endocervical canal, a biopsy was performed, and histopathological study combined with immunohistochemical analysis led to the diagnosis of the rare high-grade SEE.
In 2020, the World Health Organization (WHO) classified endometrial stromal neoplasms into four categories based on histopathological and molecular characteristics: endometrial stromal nodule, low-grade endometrial stromal sarcoma, high-grade endometrial stromal sarcoma, and undifferentiated uterine sarcoma. Histological evaluation in our patient revealed high-grade nuclear atypia with extensive vascular invasion, leading to a diagnosis of high-grade malignant neoplasm (Figures D and E). Immunohistochemical analysis showed positivity for cyclin D1, CD10, and Ki-67 (Table 1), confirming the diagnosis of high-grade SEE. Estrogen and progesterone receptors were not evaluated, but they are frequently negative in high-grade SEE, which is often associated with high expression of the BCOR protein (Batra Modi, 2020; Akaev, Yeoh, Rahimi, 2021).
Staging is performed using imaging techniques such as abdominal CT or MRI combined with chest CT and histopathological evaluation of the surgical specimen (Rauh-Hain, Del Carmen, 2013; Akaev, Yeoh, Rahimi, 2021). Treatment typically consists of total hysterectomy with bilateral salpingo-oophorectomy (BSO), with or without lymphadenectomy. Ovarian preservation can be considered in very young premenopausal patients with a desire to preserve fertility (Gadducci et al., 2023). In the case of our patient, given her youth, the tumor being confined to the cervix, and the histological subtype rarely being positive for estrogen and progesterone receptors, ovarian preservation was chosen. The risks of maintaining the organ, as well as the consequences of its removal, were discussed with the patient and her family, and together they decided on ovarian preservation with close follow-up after the procedure. However, during surgery, a macroscopic change was observed in the left ovary, leading to intraoperative removal, and subsequent pathological analysis revealed no disease in the ovary.
Regarding lymphadenectomy, there is no consensus in the literature, and it must be individualized based on the tumor subtype and disease stage. The absence of local or distant metastasis, as evidenced by imaging and free surgical margins with no angiolymphatic invasion, may support the decision not to perform lymphadenectomy, as in this case (Nasioudis et al., 2021; Rauh-Hain & Carmen, 2013).
Adjuvant therapy for high-grade SEE may be indicated depending on the disease stage, including pelvic radiotherapy with or without chemotherapy. According to Rauh-Hain and Carmen (2013), patients with early-stage disease and free margins may be followed without adjuvant therapy. In some cases, brachytherapy may be considered to better control local recurrence (Brooks et al., 2004; Azevedo et al., 2020; Akaev, Yeoh, Rahimi, 2021; Meurer et al., 2019).
In this case, a surgical approach combined with adjuvant pelvic radiotherapy was chosen; however, the patient did not undergo the treatment and lost follow-up. Active search was conducted, and contact was made with a family member, but without success. As a result, the window for adjuvant therapy was missed. The patient is currently under follow-up with the clinical and surgical oncology team, with no evidence of disease recurrence to date.
CONCLUSION
This case report highlights the importance of careful clinical attention and accurate diagnosis in young patients presenting with abnormal uterine bleeding. Furthermore, it contributes to the understanding of high-grade endometrial stromal sarcomas in unusual locations, such as the cervix, providing valuable data for a better understanding of this rare pathology. The dissemination of reports like this is essential to enhance the diagnosis and treatment of future cases.
REFERENCES:
- AKAÉV, Iolia; YEOH, Chit Cheng; RAHIMI, Siavash. Update on Endometrial Stromal Tumours of the Uterus. Diagnostics, [S.L.], v. 11, n. 3, p. 429, 3 mar. 2021. MDPI AG. http://dx.doi.org/10.3390/diagnostics11030429.
- AZEVEDO FELTZ, Carolina; LOYOLA PREST FERRUGINI, Carolina; APARECIDA TOSATO BOLDRINI, Neide. Low-grade endometrial stromal sarcoma: case report. Revista Brasileira de Pesquisa em Saúde/Brazilian Journal of Health Research, v. 23, supl_1, p. 45-51, 10 mar. 2022. Disponível em: https://doi.org/10.47456/rbps.v23isupl_1.36305. Acesso em: 20 jul. 2024.
- BATRA MODI, Kanika. Uterine sarcomas: review and update. Singapore: Springer Singapore, 2020. p. 281-295. ISBN 9789811553165. Disponível em: https://doi.org/10.1007/978-981-15-5317-2_16. Acesso em: 20 jul. 2024.
- Brooks SE, Zhan M, Cote T, Baquet CR.. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecologic Oncology, v. 93, n. 1, p. 204-208, abr. 2004. Disponível em: https://doi.org/10.1016/j.ygyno.2003.12.029. Acesso em: 20 jul. 2024.
- Capozzi VA, Monfardini L, Ceni V, Cianciolo A, Butera D, Gaiano M, Berretta R. Endometrial stromal sarcoma: a review of rare mesenchymal uterine neoplasm. Journal of Obstetrics and Gynaecology Research, v. 46, n. 11, p. 2221-2236, 23 ago. 2020. Disponível em: https://doi.org/10.1111/jog.14436. Acesso em: 20 jul. 2024.
- EAMUDOMKARN, Nuntasiri; ITARAT, Yuwadee; KLEEBKAOW, Pilaiwan; KIETPEERAKOOL, Chumnan. A Case Report of High-Grade Endometrial Stromal Sarcoma: a rare cause of abnormal uterine bleeding in a young woman. Case Reports In Obstetrics And Gynecology, [S.L.], v. 2018, p. 1-5, 28 nov. 2018. Hindawi Limited. http://dx.doi.org/10.1155/2018/5906760.
- Giuntoli RL 2nd, Garrett-Mayer E, Bristow RE, Gostout BS. Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma. Gynecologic Oncology, v. 106, n. 1, p. 82-88, jul. 2007. Disponível em: https://doi.org/10.1016/j.ygyno.2007.02.031. Acesso em: 20 jul. 2024.
- HUANG, P.-S.. Undifferentiated endometrial sarcoma of the cervix: a case report. European Journal Of Gynaecological Oncology, [S.L.], v. 40, n. 6, p. 1035, 2019. MRE Press. http://dx.doi.org/10.12892/ejgo4646.2019.
- NUGENT, Elizabeth K. et al. The value of perioperative imaging in patients with uterine sarcomas. Gynecologic Oncology, v. 115, n. 1, p. 37-40, out. 2009. Disponível em: https://doi.org/10.1016/j.ygyno.2009.06.013. Acesso em: 20 jul. 2024.
- RAUH-HAIN, J. Alejandro; CARMEN, Marcela G. del. Endometrial Stromal Sarcoma. Obstetrics & Gynecology, [S.L.], v. 122, n. 3, p. 676-683, set. 2013. Ovid Technologies (Wolters Kluwer Health). http://dx.doi.org/10.1097/aog.0b013e3182a189ac.
- USHA, M; RAU, Aarathir; SUJANI, Bk; URAVASHI, T. Low grade endometrial stromal sarcoma presenting as a cervical polyp in a young female: a rare case report. Clinical Câncer Investigation Journal, [S.L.], v. 3, n. 3, p. 257, 2014. Polaris. http://dx.doi.org/10.4103/2278-0513.132127.
- Chang K.I. Crabtree G.S, Lim-Tan S.K. Kempson R.L., Hendrickson M.R:” primary extrauterine endometrial estromal neoplasms: a clinicopathologic study of 20 cases and a review of and a review of the literature”. Int. J. Gynecolol. Pathol.,
- ABELL, Murray R.; G, Jose A. Ramirez.. Sarcomas and carcinosarcomas of the uterine cervix. Cancer, [S.L.], v. 31, n. 5, p. 1176-1192, maio 1973. Wiley. http://dx.doi.org/10.1002/1097-0142(197305)31:53.0.co;2-k.
- Hasiakos D, Papakonstantinou K, Kondi-Paphiti A, Fotiou S. Low-grade endometrial stromal sarcoma of the endocervix. Report of a case and review of the literature. Eur J Gynaecol Oncol. 2007;28(6):483-6. PMID: 18179142.
- Boardman CH, Webb MJ, Jefferies JA. Low‐grade endometrial stromal sarcoma of the ectocervix after therapy for breast cancer.Gynecol Oncol 2000;79:120‐3.
- GADDUCCI, Angiolo; MULTINU, Francesco; VITIS, Luigi Antonio de; COSIO, Stefania; CARINELLI, Silvestro; ALETTI, Giovanni Damiano. Endometrial stromal tumors of the uterus: epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecologic Oncology, [S.L.], v. 171, p. 95-105, abr. 2023. Elsevier BV. http://dx.doi.org/10.1016/j.ygyno.2023.02.009.
- MEURER, Marie; A FLOQUET,; RAY-COQUARD, I; BERTUCCI, F; AURICHE, M; A CORDOBA,; PIPERNO-NEUMANN, S; SALAS, S; DELANNES, M; CHEVALIER, T. Localized high grade endometrial stromal sarcoma and localized undifferentiated uterine sarcoma: a retrospective series of the french sarcoma group. International Journal Of Gynecologic Cancer, [S.L.], v. 29, n. 4, p. 691-698, 16 fev. 2019. BMJ. http://dx.doi.org/10.1136/ijgc-2018-000064.
- Li Y, Gong Q, Peng J, Liu Y, Jiang Y, Zhang Prognostic significance of lymphadenectomy in uterine leiomyosarcomas and endometrial stromal sarcomas: Systematic review and meta-analysis.. Eur J Obstet Gynecol Reprod Biol. 2022;279:94–101. doi: 10.1016/j.ejogrb.2022.10.013.
- Nasioudis D, Mastroyannis S, Latif N, Ko E, Haggerty A, Kim S, Morgan M, Giuntoli RL. Role of lymphadenectomy for apparent early stage uterine sarcoma; a comprehensive analysis of the National Cancer Database. Surg Oncol. 2021;38:101589. – PubMed
- MALOUF, G.G.; DUCLOS, J.; REY, A.; DUVILLARD, P.; LAZAR, V.; HAIE-MEDER, C.; BALLEYGUIER, C.; MORICE, P.; LHOMMÉ, C.; PAUTIER, P.. Impact of adjuvant treatment modalities on the management of patients with stages I–II endometrial stromal sarcoma. Annals Of Oncology, [S.L.], v. 21, n. 10, p. 2102-2106, out. 2010. Elsevier BV. http://dx.doi.org/10.1093/annonc/mdq064.
