REVASCULARIZAÇÃO PULPAR EM MOLARES INFERIORES
REVASCULARIZACIÓN PULPAR EN MOLARES INFERIORES
REGISTRO DOI: 10.69849/revistaft/ar10202409180803
Luciano Barreto Silva¹; Pedro Guimarães Sampaio Trajano dos Santos²; Rosana Maria Coelho Travassos³; Paulo Maurício de Reis Melo Júnior⁴; Ailton Coelho de Ataíde Filho⁵; Eudoro de Queiroz Marques Filho⁶; Rodolfo Scavuzzi Carneiro Cunha⁷; Juliana Perez Leyva Ataíde⁸; Nathara Killen Maciel dos Santos⁹; Advisor: Sandra Sayão Maia¹⁰.
Abstract
Objective: The aim of this study was to report a pulp revascularization case report performed on a lower molar. Methodology: The searches were carried out on Web of Science, PUBMED Central, BVS/BIREME, Scielo, Brazilian CAPES as well as Google academic portal. Twenty four articles were researched concerning the revascularization process to reach apical finalization. Knowledge from books and periodicals were also consulted, constituting the gray literature to give reliable information for this paper. In order to reinforce the basis of this study, it was decided to use the protocols of Yin (2001) and Pereira et al. (2008). Results: During the reporting process, supported by literature review, it is possible to note that for pulp revascularization to occur successfully, infection control, stimulation of local bleeding and cell activation are necessary steps. Furthermore, factors such as age, clinical cure and nutritional conditions directly influence the success rate of each case.
Keywords: Endodontics; Pulp Necrosis; Regenerative Endodontics.
Resumo
Objetivo: O objetivo deste estudo foi relatar um caso de revascularização pulpar realizada em um molar inferior. Metodologia: As buscas foram realizadas na Web of Science, PUBMED Central, BVS/BIREME, Scielo, CAPES brasileira e também no portal acadêmico Google. Foram pesquisados 24 artigos referentes ao processo de revascularização para chegar à finalização apical. Também foram consultados conhecimentos de livros e periódicos, constituindo a literatura cinza para fornecer informações confiáveis para este artigo. Para reforçar as bases deste estudo, optou-se por utilizar os protocolos de Yin (2001) e Pereira et al. (2008). Resultados: Durante o processo de relato, apoiado em revisão de literatura, é possível notar que para que ocorra a revascularização pulpar com sucesso, o controle da infecção, a estimulação do sangramento local e a ativação celular são etapas necessárias. Além disso, fatores como idade, cura clínica e condições nutricionais influenciam diretamente na taxa de sucesso de cada caso.
Palavras-chave: Endodontia; Necrose Pulpar; Endodontia Regenerativa.
Resumen
Objetivo: El objetivo de este estudio fue reportar un caso de revascularización pulpar realizada en un molar inferior. Metodología: Las búsquedas fueron realizadas en Web of Science, PUBMED Central, BVS/BIREME, Scielo, CAPES brasileña y también en el portal académico Google. Se investigaron 24 artículos sobre el proceso de revascularización para alcanzar la terminación apical. También se consultó conocimiento de libros y publicaciones periódicas, que constituyen literatura gris para proporcionar información confiable para este artículo. Para reforzar las bases de este estudio, optamos por utilizar los protocolos de Yin (2001) y Pereira et al. (2008). Resultados: Durante el proceso de informe, respaldado por una revisión de la literatura, es posible observar que para que se produzca una revascularización pulpar exitosa, el control de la infección, la estimulación del sangrado local y la activación celular son pasos necesarios. Además, factores como la edad, la curación clínica y las condiciones nutricionales influyen directamente en la tasa de éxito de cada caso.
Palabras clave: Endodoncia; Necrosis Pulpar; Endodoncia regenerativa.
1. Introduction
Much has been mentioned about traditional endodontic treatment, which uses a chemical-mechanical preparation of the root canals in order to eliminate microorganisms and pulp remains, along with the smear layer, from them. The treatment is recognized as a science, and has been used in odontology for decades. Nevertheless, it is not the best choice for immature teeth with open apex, and one of the main reasons is that the unfinished apex leaves behind very thin walls which, after obturation, may fracture due to the strong forces applied over the root. The incomplete root closure is usually caused by dental trauma, as well as root canal infections strong enough to impede mineral deposition in the tip of the apex, causing necrosis and periapical lesions. (Torabinejad, 1985).
In a recent past, the available treatment for the root apex closure has been specification, which consisted of inserting calcium hydroxide within the root canal, in order to activate odontoblasts and cementoblasts to induce apical closure. The major problem with this procedure was the formation of “squared apexes” with thin dentin walls, not to mention the long time necessary for the dentin-cementum barrier to be accomplished (Jung, 2008; Cymerman et al., 1984).
Regenerative endodontic procedures were firstly reported by Nygaard Ostby who described tissue formation in the root canal after pulp removal. The experiment was accomplished in 47 teeth, from which 35 had vital pulps and 12 had necrotic pulps. Some teeth had no bleeding induced, and served as experiment control with the specific aim of understanding whether the blood clot exerted any sort of influence for regeneration to happen. Their histologic examination showed that the teeth with vital pulp had fibrous connective tissue in 28 teeth from their samples, while cementum deposition had been found in many cases, but none of the teeth with necrotic pulps showed repair, which opened questions concerning the role of infection control. Another study brought light to this matter (Lin, 2011), explaining the difference between repair and regeneration, which definitely are not synonyms (Becerra et al., 2014; Chain et al., 2003).
As research went on, other work showed the importance of poly antibiotics pastes used within the root canals in two dental appointments for infection control (Banchs et al., 2004; Friedlander, 2009). The pastes consisted of a mixture of neomycin sulfate, polymyxin B sulfate, bacitracin, and also nystatin, with also iodoform incorporated, which allowed the continuation of the root development in pulpless teeth. The revascularization process was then accomplished in at least two sessions for immature root canal teeth. Later on, however, researchers began to use one step pulp revascularization for permanent teeth with successful results. The aim of this article was to report a case of one step pulp revascularization therapy in a lower molar (Cabeceira et al., 2023; Silva et al., 2021).
2. Methodology
For the construction of this case report, electronic searches were carried out using the following descriptors: Endodontics; Pulp Necrosis; Regenerative Endodontics. The searches were carried out on Web of Science, PUBMED Central, BVS/BIREME, Scielo, Brazilian CAPES as well as Google academic portal. Twenty two articles were researched concerning pulp revascularization to reach the apical finalization. Knowledge from books and periodicals were also consulted, constituting the gray literature to give reliable information for this paper. In order to reinforce the basis of this article, it was decided to use the protocols of Yin (2001) and Pereira et al. (2008).
3. Case Report
A healthy 15-year-old female patient searched for endodontic assistance in the endodontic department of FOR because of tooth 37 (Fig1), which clinically presented fistula and a temporary obturation.
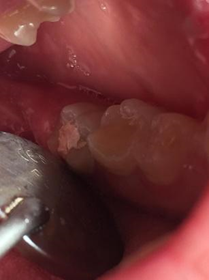
Figure 1.Clinical appearance of tooth 37 at the moment of the dental appointment.
A periapical radiograph was taken and an apical radiolucent image suggested chronic apical periodontitis. (Fig 2).
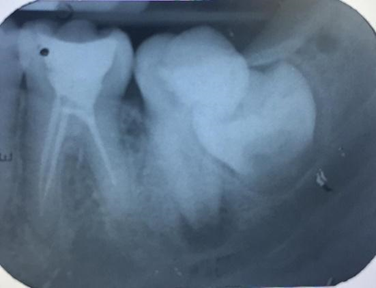
Figure 2. Tooth 37 with unfinished immature root apex and periapical lesion.
The tooth was then anesthetized, and a rubber dam was used to accomplish the coronary opening. The irrigation was softly carried out with 50 ml of 2.5% sodium hypochlorite in the pulp chamber. Later on, the root canals were irrigated with the same substance as far as the apparent working length of the tooth in the periapical radiography, up to 4 mm before the working length with a 35 K-file, in order to avoid chemical aggression to the periapex (Figure 3).

Figure 3. Exploration of the root canal with a 35 K-file 4 mm before the working length
Bleeding was then stimulated to form the blood clot which was expected to act as a framework for the neoformation of the pulp tissue, with the aid of a mechanical stimulus accomplished by a 25mm long # 35 K file, taken up to 3mm before the apparent length of the tooth (Figure 4).
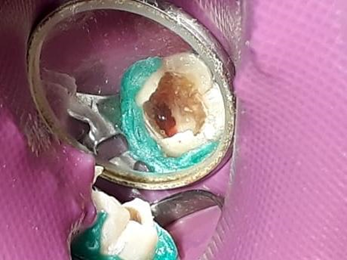
Figure 4. Bleeding stimulation for blood clot formation.
When the pulp chamber was filled, sterile gauze was used to dry the excess blood accumulated in the pulp chamber. The MTA application was then followed following the manufacturer’s protocol (Figure 5).
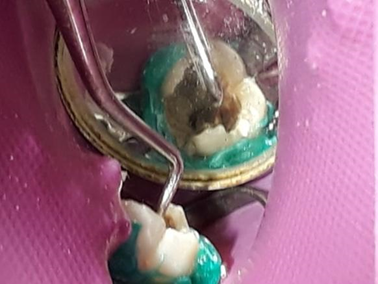
Figure 5. MTA insertion.
To complete the therapy, the tooth was restored with Bulkfill flow resin (Figure 6).
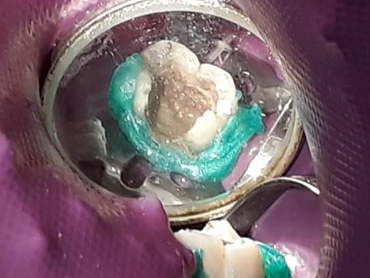
Figure 6. Tooth restored with Bulkfill flow resin.
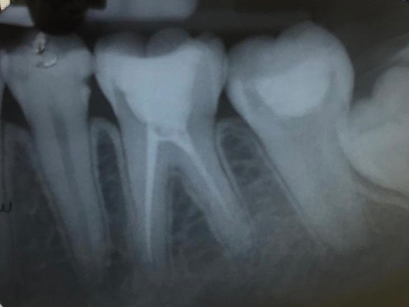
Figure 10. In April 2019, apex closure observed and dentin-like tissue formed in the ceiling of the pulp chamber.
4. Discussion
There has been a lot of controversy concerning how the denomination of pulp revascularization should be. The core of the discussion is that the term “revascularization” is only part of the process, but definitely does not correspond to the finalization of the treatment, since it also produces mineralized tissues. Similarly, it can not be named as a regenerative procedure, simply because the pulp does not end up the way it was before being infected, becoming functional as far as the point where Bioceramics were inserted within the pulp chamber, which leaves the pulp chamber obliterated. It is only expectable that the immune system play an essential role in such process (Chaplin, 2003; Clark, 1996)
One way or another, infection control seems to be the most important factor when the procedure comes into clinical procedures. Root canals infected necessarily impede the completion of the therapy, for the complex fact that immunological activity will act in the periapical region, jeopardizing healing and promoting clastic activity around and within the open apice, enhancing inflammation. Therefore, infection control is the most important step for successful therapy. Considering that the treatment worked out well, what we will have in the end is a tooth which shows part of itself newly functional, partially revascularized but not equal to what it had been before; in this sense, it can be considered as a partially regenerative outcome.
Therefore, in our point of view, this sort of therapy should be referred to as an endodontic reparative procedure(, since it involves revascularization, mineralized tissue production, and the establishment of a partial revascularized tooth. It is fundamental to remind the reader as for what concerns the role of stem cells for the finalization of the root apice (American Association of Endodontists, 2017; Couble et al., 2000). It is already a well-known fact that the adult stem cells available in the oral region come from the ectomesenchyme (About, 2000; Haeckle, 1903). It is in the embryonic development that the oral region develops a very complex interaction between embryonic cells, originating from the neural crest, right underneath the mesenchyme, with the ectoderm, responsible for the formation of the hardest hard tissue in the human body: the enamel. This interaction will promote odontogenesis (Tziafas, 2010). Part of such cells will produce the dental germ, while the other will remain as a subpopulation category of cells that will form, later on the development, the periapical area. Therefore, for revascularization to take place, it is essential the role of the stem cells present in the periapical areal. Under this point of view, for the dental apice to be finalized, it requires the differentiation and recruitment of oral stem cells, especially Dental Pulp Stem Cells (DPSCs) (Ibarretxe, 2012; Gervois et al., 2015) and Periodontal Ligament Stem Cells (PDLSCs) (Miura, 2004).
In the case report presented in this paper, the age of the patient certainly exerted a fundamental role for the apice finalization. The younger the patient is, the bigger the chances are for the therapy to work, since cellular activity in such a moment of life can not be despised. Nevertheless, infection control seems to be the most important factor for the organism to repair the area, allowing stem cells, newly former vessels and traditional adult resident cells, such as fibroblasts, to finalize immature open apices (Carmeliet et al., 2000).
5. Conclusions
Pulp revascularization seems to be a combination of infection control, professional intervention to cause bleeding, which will provide newly formed blood vessels, and cell stimulation represented by bioceramic insertion in the cervical third of the tooth involved; as well as the role of oral stem cells and traditional ones. The success rate will also depend on nutritional conditions, age, clinical healing and the due preservation of the case.
Therefore, pulp revascularization is a process that needs more research and studies, which explain how it works and what the risks and benefits are, which will be reported in case reports so that more dentists know about revascularization and thus obtain more scientific evidence.
References
American Association of Endodontists. (2017). AAE Clinical Considerations for a Regenerative Procedure. American Association of Endodontists. https://www.aae.org/specialty/wpcontent/uploads/sites/2/2017/06/currentregenerativeendodonticconsiderations.pdf
About, I., et al. (2000). Human dentin production in vitro. Exp Cel Res, 258(1), 33-41. https://doi.org/10.1006/excr.2000.4909
Banchs, F., & Trope, M. (2004). Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod, 30, 196–200. https://doi.org/10.1097/00004770-200404000-00003
Becerra, P., Ricucci, D., Loghin, S., Gibbs, J. L., & Lin, L. M. (2014). Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod, 40(1), 133–139. https://doi.org/10.1016/j.joen.2013.07.017
Cabeceira, A. L. S., Morato, G. R., & Barros, D. V. de. (2023). Pulp revascularization: A review of the literature. Research, Society and Development, 12(4), e14412441160. https://doi.org/10.33448/rsd-v12i4.41160
Carmeliet, P., & Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature, 407(6801), 249-257. https://doi.org/10.1038/35025220
Chain, J., Jones, M. K., & Tarnawski, A. A. (2003). Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. The Faseb Journal, 17, 1-19. https://doi.org/10.1096/fj.03-1232fje
Chaplin, D. D. (2003). Overview of the immunologic response. Allergy and Clinical Immunology, 111.
Clark, R. A. (1996). Wound repair. Overview and general considerations. In R. A. F. Clark (Ed.), The Molecular and Cellular Biology of Wound Repair (2nd ed., pp. 3–50). Plenum.
Couble, M. L., et al. (2000). Odontoblast differentiation of human dental pulp stem cells in explants culture. Calcif Tissue Int, 66(2), 129-138. https://doi.org/10.1007/PL00005833
Cymerman, J. J., et al. (1984). Human T lymphocyte subpopulations in chronic periapical lesions. J Endod, 10, 9-11.
Friedlander, L. T., Cullinan, M. P., & Love, R. M. (2009). Dental stem cells and their potential role in apexogenesis and apexification. International Endodontic Journal, 42(11), 955–962. doi:10.1111/j.1365-2591.2009.01622.x.
Gervois, P., Struys, T., Hilkens, P., et al. (2015). Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells and Development, 24(3), 296-311. doi:10.1089/scd.2014.0117. PMID: 25203005.
Haeckel, E. (1903). Historie de La création des êtres organisés (3rd ed.). Paris: Librairie C. Reinwald.
Ibarretxe, G., Crende, O., Aurrekoetxea, M., Garcia-Murga, V., Etxaniz, J., & Unda, F. (2012). Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration. doi:10.1155/2012/103503.
Jung, I. Y., Lee, S. J., & Hargreaves, K. M. (2008). Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. Journal of Endodontics, 34(7), 876–887. doi:10.1016/j.joen.2008.03.023.
Lin, L. M., & Rosenberg, P. A. (2011). Repair and regeneration in endodontics. International Endodontic Journal, 44(10), 889-906. doi:10.1111/j.1365-2591.2011.01915.x
Miura, M., & Shi, S. (2004). Human PDL contains stem cells that could be used to regenerate periodontal tissue. The Lancet, 364(9429), 149-155. PMID: 15246727.
Nygaard-Ostby, B., & Hjortdal, O. (1971). Tissue formation in the root canal following pulp removal. Scandinavian Journal of Dental Research, 79(5), 333-349. PMID: 5315973.
Pereira A. S. et al. (2018). Metodologia da pesquisa científica. [free e-book]. Santa Maria/RS. Ed. UAB/NTE/UFSM
Silva, H. F. da, Morais, A. P. A. G., Sampaio, G. M., Oliveira, G. H. Q., Melo Júnior, P., Maia, S. S., & Silva, L. B. (2021). Apice closure in pulp revascularization therapy: Case report and literature review. Research, Society and Development, 10(9), e21110917814. https://doi.org/10.33448/rsd-v10i9.17814
Torabinejad, M., & Kettering, J. D. (1985). Identification and relative concentration of B and T lymphocytes in human chronic periapical lesions. Journal of Endodontics, 11, 122-125.
Tziafas, D., & Kodonas, K. (2010). Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. Journal of Endodontics, 36(5), 781-9. doi:10.1016/j.joen.2010.02.006
Yin, R. K. (2001). Estudo de caso: planejamento e métodos (2a ed.). Porto Alegre: Bookman. (M. Ballejo Canto, Trad.).
¹ORCID: https://orcid.org/0000-0002-1508-4812. Universidade de Pernambuco, Brazil. E-mail: lucianobarreto63@gmail.com
²ORCID: https://orcid.org/0009-0001-5720-603X. Faculdade de Odontologia do Recife, Brazil. E-mail: pedroguimaraessampaio@gmail.com
³ORCID: https://orcid.org/0000-0003-4148-1288. Universidade de Pernambuco, Brazil. E-mail: rosana.travassos@upe.br
⁴ORCID: https://orcid.org/0000-0001-9926-5348. Faculdade de Odontologia do Recife, Brazil. E-mail: paulo.reis@upe.br
⁵ORCID: https://orcid.org/0000-0002-8105-4259. Faculdade de Odontologia do Recife, Brazil. E-mail: ailtonataide@hotmail.com
⁶ORCID: https://orcid.org/0000-0001-9794-0311. Faculdade de Odontologia do Recife, Brazil. E-mail: eudoromarques@hotmail.com
⁷ORCID: https://orcid.org/0000-0001-7110-848X. Faculdade de Odontologia do Recife, Brazil. E-mail: scavuzzi@gmail.com
⁸ORCID: https://orcid.org/0009-0000-3673-7651. Universidade de Pernambuco, Brazil. E-mail: juliana.ataide@upe.br
⁹ORCID: https://orcid.org/0009-0002-7087-2325. Faculdade de Odontologia do Recife, Brazil. E-mail: natharasantos1@gmail.com
¹⁰ORCID: https://orcid.org/0000-0001-6808-9775. Faculdade de Odontologia do Recife, Brazil. E-mail: sandrinhasayao@hotmail.com
