REGISTRO DOI: 10.5281/zenodo.10701358
Daniel Antunes Pereira¹; Jovana Dardengo Peres de Freitas Lemos²; Nicolle de Freitas Lordello³; Roberta Lúcia de Souza Teixeira4; Daila Oliveira da Rocha5; Aécio Garcia de Andrade6; Anick Martins de Andrade7; Camila Ferraz Machado8; Luiz Henrique Ferreira da Silva9; Rafaela Ohana e Silva Cristina10; Andrea de Paula Barros Araujo¹¹.
ABSTRACT
Cerebral hypoxia-ischemia is a severe condition that results in significant brain damage. This report addresses the neurological complications associated with this condition and neuroimaging findings at different times during the progression of the lesion. Patients with pre-existing conditions, such as systemic arterial hypertension (SAH) and obesity, are at greater risk of developing severe complications in response to cerebral hypoxia-ischemia. A 73-year-old man with a history of hypertension and obesity was hospitalized due to COVID-19 and experienced multiple cardiorespiratory arrests, resulting in a prolonged coma. MRIs revealed brain lesions consistent with hypoxia-ischemia, with areas of diffusion restriction and malacia in the white matter of the cerebral hemispheres. The evolution of the case showed the progression of brain lesions, with subsequent MRIs revealing sequelae of hypoxic-ischemic injury, including reduced cerebral white matter thickness and foci of hemorrhagic residue. These findings highlight the importance of continuous monitoring and early intervention in patients with cerebral hypoxia-ischemia to minimize brain damage and optimize neurological outcomes.
- Keywords: hypoxic-ischemic brain injury, Cardiac Arrest, Magnetic Resonance Image, COVID-19
INTRODUCTION
Hypoxic-ischemic brain injury (HI-BI) represents a significant challenge due to its association with oxygen deprivation to the brain, especially in cases of out-of-hospital cardiorespiratory arrest where survival rates are drastically reduced to less than 10 %(JANG; KWON, 2016). Neurological symptoms characterize this condition, including motor dysfunction and somatosensory and cognitive impairment. Notably, more than 69% of patients with HI-BI experience some degree of memory impairment, with the critical period for recovery of this function occurring within the first three months after the event. However, despite its prevalence, understanding the underlying causes of memory impairment in the early stages of HI-BI remains limited, highlighting the need for further investigation to facilitate effective rehabilitation(BUSL; GREER, 2010).
Acute Disseminated Encephalomyelitis (ADEM), classified among the monophasic demyelinating diseases of the central nervous system (CNS), represents a diagnostic challenge due to its varied clinical presentation and the absence of specific biological markers(MAHAPURE; PRABHUNE; CHOUVHAN, 2021; MARCHIONI et al., 2008). Differentiating ADEM from other neurological conditions, such as microbial infections and demyelinating diseases, represents an additional challenge due to overlapping symptoms and lack of distinct biological markers. Multiple Sclerosis (MS) is one of the main conditions that resemble ADEM, especially its multiphasic variants. However, ADEM can be distinguished from MS during the acute phase through specific clinical and laboratory findings, such as impaired consciousness, pleocytosis in the cerebrospinal fluid, and the absence of persistent oligoclonal bands. Understanding these distinctions is crucial to guide proper diagnosis and treatment(MARCHIONI et al., 2008).
The pathophysiology of hypoxic-ischemic brain injury includes a variety of mechanisms that result in cellular and tissue damage. Deprivation of oxygen and blood flow to the brain triggers a cascade of events, including a drop in brain glucose concentrations and depletion of adenosine triphosphate (ATP) and phosphocreatine, leading to irreversible neuronal damage. Ischemia time is a crucial factor in the extent and severity of damage, with more extended periods resulting in more extensive damage. Understanding these mechanisms is essential to developing effective treatment and rehabilitation strategies(BUSL; GREER, 2010; CARAMELO et al., 2023).
Management of in-hospital cardiac arrest is critical to improving clinical and neurological outcomes. International guidelines recommend the use of targeted temperature management (TTM) and urgent coronary interventions to optimize survival and minimize neurological damage. Recent studies have demonstrated that TTM at 36°C can provide similar benefits to TTM at 33°C, but the optimal cooling duration is still being debated. Maintaining the patient’s temperature within a specific range for at least 24 hours after cardiac arrest has been associated with better clinical outcomes(CARAMELO et al., 2023; KIRKEGAARD et al., 2017).
The brain’s susceptibility to damage from blood deprivation is well documented, especially in animal model studies. The duration and extent of ischemic damage are directly related to the interruption of blood flow to the brain. After just a few minutes of ischemia, irreversible cellular damage occurs, affecting neurological function. Treatment and rehabilitation strategies must consider these pathophysiological complexities to maximize the chances of recovery(BUSL; GREER, 2010).
A delay between the initial insult and the manifestation of cellular damage characterizes ischemic cell death. This delay varies depending on the duration and type of insult and the brain region affected. Studies have shown that after circulatory arrest, up to 95% of brain tissue can be damaged after 15 minutes of ischemia. Understanding the mechanisms underlying ischemic cell death is essential for developing targeted therapeutic strategies and improving clinical outcomes in patients with hypoxic-ischemic brain injury(CHAWLA, 2020; POPESCU, 2021).
CASE REPORT
A 73-year-old man with no previous neurological history had a medical history of systemic arterial hypertension (SAH) and obesity. He was hospitalized for a period of four months (from April 12, 2021, to August 3, 2021) due to a COVID-19 infection, the diagnosis of which was established clinically by rapid tests and through imaging tests. During hospitalization, his health condition deteriorated, mainly with respiratory and cardiovascular impairment, requiring transfer to the intensive care unit (ICU), where he experienced several cardiorespiratory arrests. After resuscitation, which lasted approximately 15 minutes, he fell into a coma with no response to stimuli.
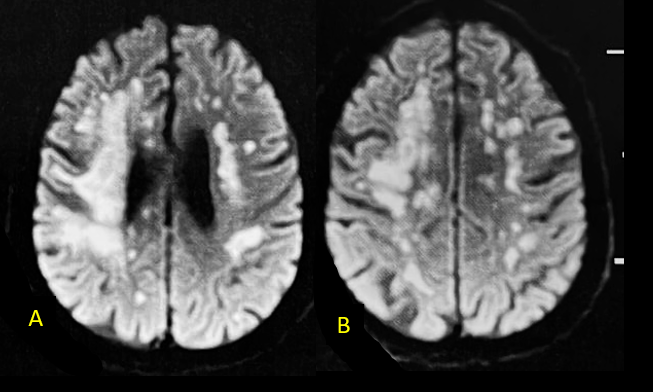
One month after his hospital discharge (on September 24, 2021), a FLAIR-weighted MRI revealed diffusion-restricted lesions in the white matter of the cerebral hemispheres, distributed in a topography suggestive of a vascular border zone (A and B). At that time, acute disseminated encephalomyelitis (ADEM) was suspected, leading to the administration of pulse therapy. He was discharged after four months of hospitalization. At the current moment, he remains without the ability to communicate or socially interact, just following with his eyes. (Figure 1)
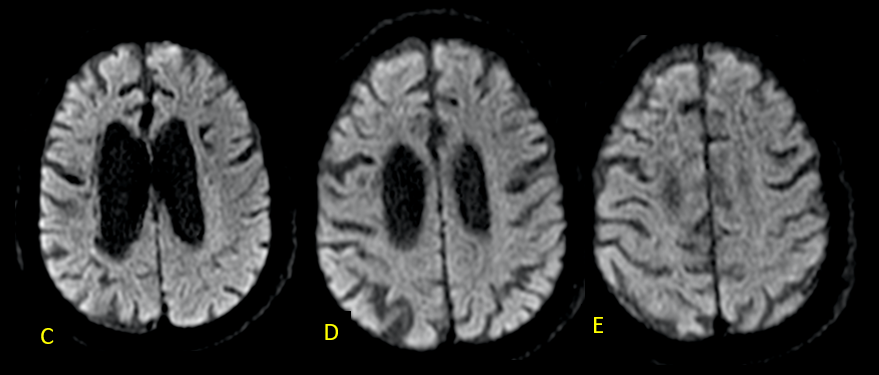
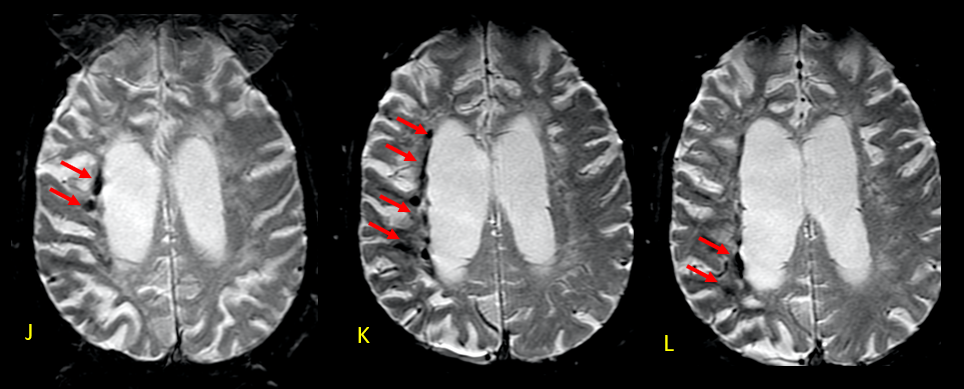
A recent MRI, dated November 6, 2023 (two years and two months after the previous one), no longer reveals diffusion restriction (C-E). However, in FLAIR-weighted axial sections, a reduction in the thickness of the cerebral white matter is observed, associated with hypersignal, suggestive of sequelae of hypoxic-ischemic injury related to cardiorespiratory arrests during hospitalization. Lesions are more pronounced in the right hemisphere, where the decrease in white matter thickness is most significant, and there is evidence of interpunctuated areas of malacia (arrows in F). (Figures 2 and 3)
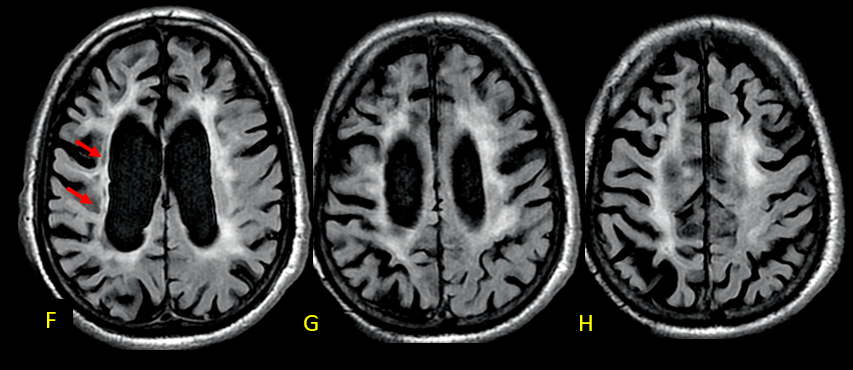
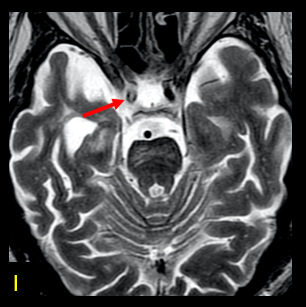
The greater severity of the involvement on the right is attributed to thrombosis of the internal carotid artery on that side (arrow in I-axial T2). In the weighted susceptibility sequence (SWI), multiple foci of hemorrhagic residue are visualized in the white matter adjacent to the body of the right lateral ventricle (arrows), indicative of sequelae of hypoxia/ischemia. (Figures 4 and 5)
DISCUSSION
The neurological condition presented by the 73-year-old patient, with a previous history of systemic arterial hypertension (SAH) and obesity, reveals a complex interaction between clinical and neurological factors. Prolonged hospitalization due to COVID-19 was investigated in several cardiorespiratory arrests, triggering a long coma and serious injuries. MRI initially showed diffusion restriction in the white matter of the dangerous hemispheres, possibly possible involvement of acute disseminated encephalomyelitis (ADEM), a rare but specific neurological complication of COVID-19 infection(AGRAWAL et al., 2022; MAHAPURE; PRABHUNE; CHOUVHAN, 2021; PAOLILO et al., 2020). Administration of pulse therapy was initiated, but complete recovery was not achieved, as evidenced by the persistence of the coma and the inability to communicate or socially interact.
The subsequent MRI, performed two years and two months after the initial hospitalization, revealed an evolution in the characteristics of the skin lesions. Although there was no more extended restriction to diffusion, a reduction in the thickness of the cerebral white matter and areas of hypersignal were observed, indicative of sequelae of hypoxic-ischemic injury related to cardiorespiratory arrests during hospitalization. These changes were more pronounced in the right hemisphere, attributed to thrombosis of the internal carotid arteries on that side. Furthermore, the presence of multiple foci of hemorrhagic discharge in the white matter adjacent to the body of the right lateral ventricle suggests additional complications of hypoxia/ischemia(BUSL; GREER, 2010; CARAMELO et al., 2023; RAJAJEE et al., 2023; SANDRONI; D’ARRIGO; NOLAN, 2018a).
Discussion of these clinical and radiological findings raises important questions about the management and prognosis of patients with hypoxic-ischemic injuries. Studies emphasize the importance of ischemia time in the extent and severity of damage caused, with longer periods associated with neurological injuries. Furthermore, early identification of risk factors, such as arterial thrombosis, can influence prevention and treatment strategies. Administration of neuroprotective therapies, such as controlled temperature, has been investigated as an approach to minimize physical harm and improve clinical outcomes after cardiopulmonary arrest(KIM et al., 2014; KIRKEGAARD et al., 2017; SANDRONI et al., 2014).
Recent studies also highlight the complexity of neurological recovery after hypoxic-ischemic injuries, with a variety of factors influencing the patient’s response to treatment. Understanding the underlying pathophysiological mechanisms is crucial to guide therapeutic and prognostic interventions. Furthermore, the identification of specific biomarkers can help in tracking and monitoring patients at risk of developing neurological complications after hypoxia/ischemia events. These considerations highlight the continued need for clinical and translational research to improve the approach and management of patients with specific hypoxic-ischemic injuries(DIETRICH; BRAMLETT, 2017; KIRKEGAARD et al., 2017; RAJAJEE et al., 2023; SANDRONI; D’ARRIGO; NOLAN, 2018b).
CONCLUSION
This report illustrates the challenges faced in managing and understanding neurological complications resulting from hypoxia/ischemia events, especially in the context of COVID-19 and cardiorespiratory arrests. Radiological evolution over time reflects brain injuries’ complexity and long-term consequences, emphasizing the importance of early and multidisciplinary therapeutic strategies. Furthermore, the discussion highlights the need for a holistic approach in the evaluation and treatment of patients with hypoxic-ischemic brain injuries, integrating both medical interventions and multidisciplinary support to optimize neurological and functional outcomes. A deeper understanding of the mechanisms underlying these injuries, combined with a patient-centered approach, is essential to guide clinical practice and promote better outcomes for individuals affected by these complex neurological conditions.
REFERENCES
AGRAWAL, S. et al. Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profiles of acute vascular injury, inflammation, and age-linked chronic brain diseases. Acta neuropathologica communications, v. 10, n. 1, 1 dez. 2022.
BUSL, K. M.; GREER, D. M. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation, v. 26, n. 1, p. 5–13, 2010.
CARAMELO, I. et al. Biomarkers of hypoxic-ischemic encephalopathy: a systematic review. World journal of pediatrics : WJP, v. 19, n. 6, p. 505–548, 1 jun. 2023.
CHAWLA, D. Biomarkers for Prognostication in Hypoxic-Ischemic Encephalopathy. Indian journal of pediatrics, v. 87, n. 10, p. 777–778, 1 out. 2020.
DIETRICH, W. D.; BRAMLETT, H. M. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: Experimental and clinical experience. Brain Circulation, v. 3, n. 4, p. 186, 2017.
JANG, S. H.; KWON, H. G. Neural injury of the Papez circuit following hypoxic–ischemic brain injury: A case report. Medicine, v. 95, n. 44, 2016.
KIM, F. et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA, v. 311, n. 1, p. 45–52, 1 jan. 2014.
KIRKEGAARD, H. et al. Targeted Temperature Management for 48 vs 24 Hours and Neurologic Outcome After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA, v. 318, n. 4, p. 341–350, 25 jul. 2017.
MAHAPURE, K.; PRABHUNE, A.; CHOUVHAN, A. COVID-19-Associated Acute Disseminated Encephalomyelitis: A Systematic Review. Asian journal of neurosurgery, v. 16, n. 3, p. 457–469, set. 2021.
MARCHIONI, E. et al. Acute disseminated encephalomyelitis. Neurological Sciences, v. 29, n. SUPPL. 2, set. 2008.
PAOLILO, R. B. et al. Acute Disseminated Encephalomyelitis: Current Perspectives. Children (Basel, Switzerland), v. 7, n. 11, 1 nov. 2020.
POPESCU, C. Hypoxic-Ischemic Injury of Basal Ganglia Associated with the COVID-19 Infection: Case Report. Case reports in neurology, v. 13, n. 3, p. 668–671, 4 out. 2021.
RAJAJEE, V. et al. Guidelines for Neuroprognostication in Comatose Adult Survivors of Cardiac Arrest. Neurocritical care, v. 38, n. 3, p. 533–563, 1 jun. 2023.
SANDRONI, C. et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive care medicine, v. 40, n. 12, p. 1816–1831, 21 nov. 2014.
SANDRONI, C.; D’ARRIGO, S.; NOLAN, J. P. Prognostication after cardiac arrest. Critical Care, v. 22, n. 1, p. 1–9, 5 jun. 2018a.
SANDRONI, C.; D’ARRIGO, S.; NOLAN, J. P. Prognostication after cardiac arrest. Critical care (London, England), v. 22, n. 1, 5 jun. 2018b.
¹Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: danielantunespi@gmail.com
²Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: jovanadardengo@hotmail.com
³Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: nicolle-lordello@hotmail.com
4Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: robertalteixeira@gmail.com
5Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: rochadaila66@gmail.com
6Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: aecioandrade@icloud.com
7Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: anickmartins@gmail.com
8Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: medcamilaferraz@gmail.com
9Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: luizhenriqueferreira01@gmail.com
10Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: rafaelaohana2016@outlook.com
¹¹Discente do Curso Superior de Medicina da Universidade Iguaçu Campus Nova Iguaçu e-mail: andreaaraujo9619@yahoo.com.br
