REGISTRO DOI:10.5281/zenodo.10357051
Ruy Guimaraes Xavier dos Santos1
Carlos Mario Gonzalez Zambrano2
Fernando Carmona Dinau3
Isabelly do Lira Ladislau4
Noeme Sousa Rocha5
Abstract
Primary osteosarcoma (OSA) is the most common primary bone tumor in large dog breeds and one of the most aggressive, characterized by rapid progression. The most affected bones are the long bones of the appendicular skeleton, including the radius, ulna, humerus, femur, tibia, and fibula. A literature review was conducted to gather the most accurate and recent information on OSA, including findings and updates. With the information obtained, a survey of cases of primary osteosarcoma diagnosed in dogs from 2015 to 2020 at the Veterinary Pathology Service of the Veterinary Hospital of FMVZ – UNESP, Botucatu Campus (Júlio de Mesquita Filho) was performed. Subsequently, a comparison was made between the data obtained from cytopathological examinations of these animals, a comparison of data obtained from histopathological examinations of these animals, and finally, a comparison of data obtained from both cytopathological and histopathological examinations. The intention in these three stages is to identify common characteristics of those affected by OSA and, thus, establish a retrospective profile capable of distinguishing dogs that may be in the early stages of primary osteosarcoma or identifying dogs predisposed to developing this type of tumor. A considerable number of mixed-breed dogs were diagnosed with OSA, leading to a classification based on weight. Inconsistencies were found between the results of this research and those of other studies conducted by colleagues.
Keywords: Osteosarcoma; Cytopathology; Histopathology; Dogs; Large and Giant Breeds.
Introduction
Osteosarcoma is the most common primary bone tumor in large dog breeds. It represents 85% of primary skeletal tumors, with 75% occurring in the appendicular skeleton (1). Although it has a relatively low global incidence in humans (0.3 per 100,000 per year), it accounts for approximately 15% of pediatric tumors (2, 3). Like other sarcomas, osteosarcomas arise from the malignant transformation of mesenchymal stem cells (MSCs) or their derived cell types along the osteoblastic lineage (4).
Osteosarcoma is characterized by significant phenotypic and genomic heterogeneity, and few recurrent genetic changes have been reported (5). Osteosarcoma exhibits a complex karyotype with high genetic and chromosomal instability, resulting in multiple rearrangements across the genome (6). Identified genetic markers have been associated with treatment response and prognosis, making them promising candidates for clinical application (7).
A distinction between osteosarcoma and other non-osteogenic malignant bone tumors is the presence of osteoid. Osteosarcoma is the only tumor that forms osteoid, while tumors like chondrosarcoma and fibrosarcoma produce a cartilaginous matrix alongside a fibrous stroma. Another difference is the cell of origin: osteosarcoma originates from osteoblasts, while other tumors originate from chondroblasts, chondrocytes, fibroblasts, except for osteoma, which also originates from osteoblasts. Additionally, among these tumors, malignancy generally leads to late metastasis during the clinical course, whereas osteosarcoma presents early pulmonary metastatic activity. (9).
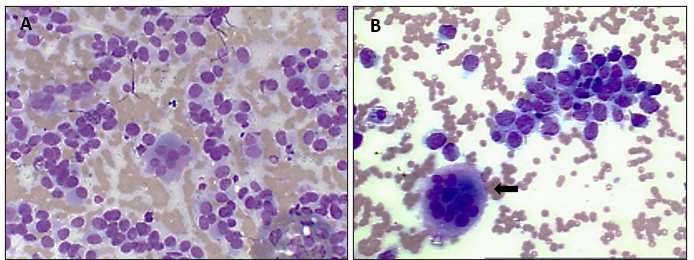
Figure 1 – (A) and (B) cytopathological examinations, where osteoblasts and osteoclasts (black arrow) can be observed within the matrix; (B) shows a scarcity of extracellular matrix. Giemsa stain, 40X
Typical clinical presentation is observed in dogs around eight years of age, with an average weight of over 18 kg. The most frequent locations affectedare the radius, distal femur, humerus, or proximal tibia. X-rays detect abnormalities in bone density and a periosteal reaction to sunlight (10, 11, 12, 13). Appendicular osteosarcoma accounts for up to 90% of cases (1).
Extra-skeletal presentation is rare but has been reported as a primary tumor in mammary tissue, subcutaneous tissue, spleen, intestine, liver, kidneys, vagina, eyes, adrenal gland, gastric ligament (14), and heart (15). Initially, palpation may not detect obvious lesions, and X-rays may show subtle changes. As the patient’s condition progresses, swelling and lameness worsen rapidly, and the lesion becomes painful to the touch. Without treatment, progressive erosion of the cortical bone can lead to pathologic fracture of the affected limb. The clinical course can be very short, often ranging from 1 to 3 months (16). Presumptive diagnosis of these tumors can be made by plain radiography, and definitive diagnosis is made by histopathology, although it can be specified with cytological analysis (17).
The pattern of bone lysis can be:
Geographic pattern: Focal areas of lysis tend to have well-defined margins, the cortex is expanded but not lytic. These are the less aggressive forms, which can be caused by a bone cyst or an abscess.
Permeative or moth-eaten pattern: There is moth-eaten lysis, well-defined areas, and multiple lyses of variable size, the cortex may or may not be lytic. This type of lesion is aggressive and is typically seen in bone tumors and infections.
Mixed or permeative pattern: This is the most aggressive pattern of bone lysis, where poorly defined focal areas of osteolysis are present throughout the bone region (18).
It is unclear whether pain is simply an indicator of early death associated with osteosarcoma or if there is a true mechanistic link between pain and cancer progression. One consideration is that pronociceptive ligands produced during tumor progression that lead to the generation of pain signals (e.g., nerve growth factor, endothelin-1, prostaglandin E2, etc.) may also promote aggressive tumor behaviors (19).
Diagnosis is made, and treatment is planned by combining imaging and histopathological findings (Figure 2). Biopsy should be extensive to avoid confusion with reactive bone tissue, osteomyelitis processes, and, especially, to classify the different tumor variants (20). An inconvenience when performing bone biopsy is pathological fractures due to bone fragility. For this reason, it is necessary to perform cytology through fine needle aspiration to address a diagnosis (21).
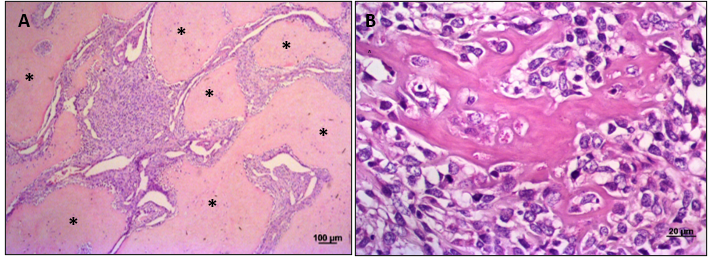
Figure 2. The main features in the histopathology of osteosarcoma are: (A) Islands of osteoid matrix (asterisk) surround the neoplastic infiltrate of osteoblasts. (B) Presence of osteoid, hyperplastic osteoblasts with morphological variations such as anisocytosis and anisocariosis, and areas of mineralization that commonly infiltrate the surrounding tissue. (H&E stain. 10x and 40x, respectively).
Treatment is not the same for all patients; it depends on the disease stage, the presence or absence of metastases, the patient’s quality of life, and economic availability. Treatment options should consider pain management and the prevention of pathological fractures.
Treatment alternatives include amputation surgery, stabilization surgery, chemotherapy, or a combination of oncologic or stabilizing surgery with chemotherapy. Radiation therapy is generally ineffective due to low radiosensitivity, slow response, and poor disease remission. However, it can be used as palliative treatment for pain reduction, as an adjuvant to surgery, or as post-surgical radiation therapy in cases where complete removal is not feasible.
Apendicular osteosarcoma is relatively resistant to radiation therapy, but it has shown significant results in reducing local pain, with survival rates similar to limb amputation (22). The International Association for the Study of Pain (IASP) defines pain as an unpleasant emotional and sensory experience associated with actual or potential tissue damage or described in terms of such damage.
Physiologically, pain has a protective role, manifested through a motor response that tends to avoid harm in the face of various noxious stimuli and is stored in the animal’s memory. However, when the stimulus is prolonged and not treated promptly, pain becomes a pathological response and can become the focus of the disease. However, Karnik et al. (2012) disagree and suggest that amputation or resection of the affected bone alone rarely leads to a definitive solution, as metastasis recurrence often occurs. Chemotherapy with multiple drugs and aggressive surgeries may have increased a dog’s chances of survival. However, new therapies for OSA remain uncertain, despite significant efforts to introduce unprecedented therapeutic advances (23, 24). According to Daleck, Fonseca, and Canola (2002), this malignant neoplasm tends to develop in bones that support the greatest weight in the canine skeleton, particularly in areas near the epiphyseal plates with delayed closure (as commonly occurs in larger and giant dog breeds, and rarely in smaller breeds).
Therefore, larger animals have a predisposition to experience numerous minor traumas in the metaphyseal regions, areas that have heightened cellular activity. Consequently, sensitizing cells in the above-mentioned region can give rise to malignant tumors by stimulating cell proliferation, triggering mitosis, and thereby increasing the likelihood of mutated cells emerging. Additionally, there have been reports of osteosarcoma in areas of bones where fractures previously occurred and had metal implants for treatment, which subsequently developed chronic osteomyelitis.
This also occurs in fractures that were not internally fixed. The third theory reported by Daleck, Fonseca, and Canola (2002) suggests that ionizing radiation may induce OSA. In an experiment, approximately 3.5% of dogs exposed to radiation therapy for the treatment of soft tissue sarcomas subsequently developed osteosarcoma in areas exposed to the radiation treatment. The last theory of Daleck, Fonseca, and Canola (2002) states that hormones and cytokines can cause the tumor by acting on bone growth areas and stimulating excessive activity in stem cells, increasing the likelihood of differentiated cell occurrence.
Unfortunately, mortality within 2 years after diagnosis exceeds 90% (24, 25). According to London et al. (2015) and Schmidt et al. (2016), the average patient’s survival can reach 5 months if only amputation is performed, and it can extend to 12 months when chemotherapy is included in the treatment. Virgílio et al. (2020) asserts that combining amputation and chemotherapy offers a 40% chance of survival up to 1 year, with a 20% chance of survival up to 2 years. While the exact etiology of OSA is unknown, there is suspicion regarding some predisposing factors: age, castration, height, weight, gender, intense physical exercise, rapid growth, center of gravity, and osteosynthesis. Each of these factors’ involvement in primary canine osteosarcoma has been mentioned in the literature, although their influence has not been definitively proven. However, age, castration, and breed show strong evidence of being predisposing factors (26, 27).
Daleck, Fonseca, and Canola (2002) propose various theories about the etiology of this malignant cancer. The first theory suggests that OSA has a virus as its etiological agent because it can occur in puppies born from the same mother and can be experimentally induced in canine fetuses through the injection of cells derived from this tumor. However, it is emphasized that no virus that could have initiated the development of osteosarcoma has been isolated.
This data collection will allow us to identify the retrospective cytopathological and histopathological profile of osteosarcoma diagnosed through two different techniques, in order to obtain data that enable a better understanding of disease predisposition, with the aim of increasing the likelihood of early diagnosis.
Materials and Methods
Animal Ethics Committee
This study was approved by the Animal Ethics Committee (CEUA) under Protocol No. 0087/2021 at the Faculty of Veterinary Medicine and Animal Science (FMVZ) of UNESP in Botucatu.
To gather data on osteosarcoma, a search was conducted in the computerized system, including requests and results of cytological and histopathological examinations performed at the Veterinary Hospital of FMVZ-UNESP in Botucatu over six years, from 2015 to 2020.
From the medical history, the following data were acquired
Age, breed, gender, and the location of the lesion. The cytological examination provided the diagnosis as well as the grading, indicating the degree of tumor aggressiveness. Subsequently, this result was compared with the histopathological examination to identify similarities or differences.Quality control was also carried out, from proper requisition filling to microscopic results.
Finally, the data were presented in tables, and suitable cytological and histopathological request and result templates were developed for the investigation of bone tumors in animals.
The total number of cytological examinations performed during the years in question included patients from other animal species besides dogs, such as sheep, goats, domestic cats, horses, donkeys etc. It is important to emphasize that in cytological examinations, there were also cases where requests were submitted, and therefore, control numbers were generated, but the owners later canceled these examinations. These canceled exams are included in the annual total.
Results
According to the survey of osteosarcoma cases in dogs through cytological and histopathological examinations, 14 patients were found to have this type of cancer through cytological examinations, and 34 patients were diagnosed with it through histopathological examinations. Approximately 7,000 cytological examinations and almost 3,000 histopathological examinations were performed on various species from 2015 to 2020. The relevant findings of this data survey are presented in Tables 1, 2, and 3.
Table 1. Cytological and Histopathological Examinations between 2015 and 2020
Year Osteosarcoma diagnosed by cytology Total Cytological Examinations Osteosarcoma diagnosed by histopathology Total histopathologycal Examinations 2015 4 1037 5 264 2016 3 1283 6 327 2017 0 985 0 303 2018 2 1078 8 469 2019 4 1181 13 632 2020 1 499 2 232
Table 2. Breeds Diagnosed with Osteosarcoma
Bread Diagnosed through Hystopathological Diagnosed through cytopathology Rottweiler 4 5 German Shepherd 1 N Boxer 1 N Pit Bull 2 N Golden Retriever 2 1 Labrador 1 N Saint Bernard 2 3 Airedale Terrier 1 N Poodle 1 N Mixed Breed 18 5
Table 3. Age of Diagnosed Animals
Age Range of Dogs Diagnosed dogs through cytopathology Diagnosted through histopathology Younger than 7 years of age 5 11 Between 7 and 10 years of age 6 9 Over 10 years of age 3 13
Discussion
This finding significantly exceeds the estimate provided by Virgilio et al (30), who claimed an incidence of 13,900 dogs diagnosed with primary osteosarcoma per 100,000. It is essential to consider that Virgilio et al’s study was conducted through a joint research effort involving six veterinarians working in three different cities in France and Belgium, i.e., European countries with different realities from Brazil.
This analysis of osteosarcoma cases in dogs also reveals that the vast majority of affected dogs were adults or in more advanced life stages. This contradicts the claims made by Daleck, Fonseca, and Canola (2002) and Virgilio et al (2020), who stated that primary osteosarcoma typically occurs in individuals aged 7 to 10 years. Once again, it should be emphasized that the research conducted by these colleagues was not carried out in Brazil, which has a different reality compared to first-world countries.
The high number of mixed-breed dogs in Brazil makes it more challenging to identify potential occurrences. Research, including that conducted in this project, has shown that large and giant breeds are more susceptible to this type of tumor. For instance, breeds like St. Bernard and Golden Retriever, which are not typically found in Brazilian soil due to their preference for cold climates and/or higher-income owners, as the puppies are sold at prices that a majority of the Brazilian population cannot afford.
Based on the weight criteria for size categories in dogs defined by Gallitelli et al (2001), one can attempt to classify each of the mixed-breed dogs into a particular size category. However, this remains an assumption as it is impossible to determine whether any of these mixed-breed dogs are morbidly obese or undernourished, for example. The 5 mixed-breed dogs vary significantly in weight.
Hence, it is of paramount importance to categorize mixed-breed dogs according to their weight in size categories for breeds. This will help determine whether the mixed-breed dog falls within the typical parameters for developing osteosarcoma seen in large and giant-sized dogs.
Unfortunately, the cytological examinations conducted by the Pathology Department at the Veterinary Hospital do not include the measurement of the dog’s height. We know that among the 9 purebred dogs, 6 belong to large breeds and 3 to giant breeds. However, we cannot be certain of the size of a mixed-breed dog.
Hence, mixed-breed dogs appear to be an excellent model for studying primary osteosarcoma and, in the future, for further exploration from a molecular perspective and using more advanced techniques. This could help improve the therapeutic approach and the quality of life for the patients.
The year 2020 should be analyzed with special attention since the number of examinations processed for both techniques decreased by an average of 50%, and the number of diagnosed cases also appeared to be lower. This can be explained by the lockdown period caused by the Sars CoV 2 (COVID19) pandemic.
Conclusions
Based on this research, it can be concluded that osteosarcoma is indeed the most common primary tumor in large and giant breed dogs, even when studying a low-income population. This suggests that the breed information provided by the animal’s owner does not always align with national and international literature data. Thus, it can be concluded that pure breed does not seem to be directly correlated with the incidence of this tumor.
During the period covered by this survey, we can observe higher sensitivity in the histopathology technique. The sensitivity of cytological examination is more closely linked to pre-analytical factors such as the skill of the professional who obtained the sample, the presence or absence of bone cortex, the size of the needle used, and the handling of the collected specimen. Nevertheless, this technique is cost-effective, minimally invasive, with few contraindications and complications.
References
- EHRHART, N. P.; RYAN, S. D.; FAN, T. M. Tumors of the Skeletal System. In: Withrow and MacEwen’s Small Animal Clinical Oncology: Fifth Edition. Elsevier Inc., 2013, p. 463-503. https://doi.org/10.1016/B978-1-4377-2362-5.00024-4
- SMELAND, S.; BIELACK, S. S.; WHELAN, J., et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer, 2019;109:36-50. doi:10.1016/j.ejca.2018.11.027
- GRÜNEWALD, T. G.; ALONSO, M.; AVNET, S., et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol Med, 2020;12(11):e11131. doi:10.15252/emmm.201911131
- GAMBERA, S.; ABARRATEGI, A.; RODRÍGUEZ-MILLA, M. A., et al. Role of Activator Protein-1 Complex on the Phenotype of Human Osteosarcomas Generated from Mesenchymal Stem Cells. Stem Cells, 2018;36(10):1487-1500. doi:10.1002/stem.2869
- PERRY, J. A.; KIEZUN, A.; TONZI, P., et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A, 2014;111(51):E5564-E5573. doi:10.1073/pnas.1419260111
- BEHJATI, S.; TARPEY, P. S.; HAASE, K., et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat Commun, 2017;8:15936. doi:10.1038/ncomms15936
- HATTINGER, C. M.; PATRIZIO, M. P.; LUPPI, S.; SERRA, M. Pharmacogenomics and Pharmacogenetics in Osteosarcoma: Translational Studies and Clinical Impact. International Journal of Molecular Sciences, 2020;21(13):4659. https://doi.org/10.3390/ijms21134659
- DALECK, C. R.; FONSECA, C. S.; CANOLA, J. C. Osteossarcoma canino – revisão. “Canine osteosarcoma – review.” Rev. Educ. Cont. Med. Vet. Zootec., 1º de dezembro de 2002, 5(3), p. 233-42. Disponível em
- ZHENG, B.; SONG, K.; SUN, L., et al. Siglec-15-induced autophagy promotes invasion and metastasis of human osteosarcoma cells by activating the epithelial-mesenchymal transition and Beclin-1/ATG14 pathway. Cell Biosci, 2022;12(1):109. doi:10.1186/s13578-022-00846-y
- CARMONA, L. A.; SANTOSCOY, M. C. Protocolo diagnóstico y terapéutico en perros sospechosos de osteosarcoma (análisis retrospectivo de 28 casos clínicos). Vet Mex, 2006;37(1):79-95. Disponível em
- BERG, J. Canine Osteosarcoma: Amputation and Chemotherapy. Veterinary Clinics of North America: Small Animal Practice, 1996;26(1):111-121. doi:10.1016/S0195-5616(96)50010-0
- SGALAMBRO, F.; ZUGARO, L.; BRUNO, F., et al. Interventional Radiology in the Management of Metastases and Bone Tumors. J Clin Med, 2022;11(12):3265. doi:10.3390/jcm11123265
- CAZZATO, R. L.; ARRIGONI, F.; BOATTA, E., et al. Percutaneous management of bone metastases: state of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR). Radiol Med, 2019;124(1):34-49. doi:10.1007/s11547-018-0938-8
- OKA, T.; MATSUZAKI, K.; IZUMI, H., et al. Osteosarcoma of the bladder: a case report. J Med Case Rep, 2022;16(1):118. doi:10.1186/s13256-022-03346-2
- SHIRLOW, A.; BORGEAT, K.; HAYWARD, N., et al. Metastatic osteosarcoma tumor thrombus in a Cavalier King Charles Spaniel presenting with dyspnea. Journal of Veterinary Cardiology, 2022;41:209-215. doi:10.1016/j.jvc.2022.03.003
- CHAIB, B.; MALHOTRA, K.; KHOO, M.; SAIFUDDIN, A. Pathological fracture in pediatric bone tumors and tumor-like lesions: A predictor of benign lesions?. Br J Radiol, 2021;94(1125):20201341. doi:10.1259/bjr.20201341
- COUTO, J. I.; BEAR, M. D.; LIN, J., et al. Biologic activity of the novel small molecule STAT3 inhibitor LLL12 against canine osteosarcoma cell lines. BMC Vet Res, 8, 244, 2012. https://doi.org/10.1186/1746-6148-8-244
- PAN, D.; LIU, R.; ZHENG, B., et al. Using Machine Learning to Unravel the Value of Radiographic Features for the Classification of Bone Tumors. Biomed Res Int, 2021;2021:8811056. doi:10.1155/2021/8811056
- SHOR, S.; FADL-ALLA, B. A.; PONDENIS, H. C., et al. Expression of nociceptive ligands in canine osteosarcoma. J Vet Intern Med, 2015;29(1):268-275. doi:10.1111/jvim.12511
- HARPER, K.; SATHIADOSS, P.; SAIFUDDIN, A.; SHEIKH, A. A review of imaging of surface sarcomas of bone. Skeletal Radiol, 2021;50(1):9-28. doi:10.1007/s00256-020-03546-1
- ARIIZUMI, T.; KAWASHIMA, H.; YAMAGISHI, T., et al. Diagnostic accuracy of fine needle aspiration cytology and core needle biopsy in bone and soft tissue tumor: A comparative study of the image-guided and blindly performed procedure. Ann Diagn Pathol, 2022;59:151936. doi:10.1016/j.anndiagpath.2022.151936
- MARTIN, T.; GRIFFIN, L.; CUSTIS, J., et al. Resultado y pronóstico del osteosarcoma apendicular canino tratado con radioterapia corporal estereotáctica en 123 perros. Vet Comp Oncol, (2021) 19:284–94. doi: 10.1111/vco.12674
- FENGER, J. M.; LONDON, C. A.; KISSEBERTH, W. C. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J, 2014;55(1):69-85. doi:10.1093/ilar/ilu009
- LONDON, C. A.; GARDNER, H. L.; MATHIE, T., et al. Impact of Toceranib/Piroxicam/Cyclophosphamide Maintenance Therapy on Outcome of Dogs with Appendicular Osteosarcoma following Amputation and Carboplatin Chemotherapy: A Multi-Institutional Study. PLoS One, 2015;10(4):e0124889. doi:10.1371/journal.pone.0124889
- GARDNER, H. L.; SIVAPRAKASAM, K.; BRIONES, N., et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun Biol, 2, 266, 2019. https://doi.org/10.1038/s42003-019-0487-2
- RU, G.; TERRACINI, B.; GLICKMAN, L. T. Host related risk factors for canine osteosarcoma. The Veterinary Journal, 1998;156:31-39. https://doi.org/10.1016/S1090-0233(98)80059-2
- SZEWCZYK, M.; LECHOWSKI, R.; ZABIELSKA, K. What do we know about canine osteosarcoma treatment? Review. Vet Res Commun, 2015;39(1):61-67. doi:10.1007/s11259-014-9623-0
- KARNIK, K. S.; SAMII, V. F.; WEISBRODE, S. E., et al. Accuracy of computed tomography in determining lesion size in canine appendicular osteosarcoma. Vet Radiol Ultrasound, 2012;53(3):273-279. doi:10.1111/j.1740-8261.2012.01930.x
- SCHMIDT, A. F.; GROENWOLD, R. H.; AMSELLEM, P., et al. Which dogs with appendicular osteosarcoma benefit most from chemotherapy after surgery? Results from an individual patient data meta-analysis. Prev Vet Med, 2016;125:116-125. doi:10.1016/j.prevetmed.2015.10.016
- VIRGILIO, F.; CARATY, J.; HASSOUN, R.; HAMAIDE, A.; MEHEUST, P.; FARNIR, F. Evaluations of phylogenetic proximity in a group of 67 dogs with osteosarcoma: a pilot study. *Journal of Veterinary Healthcare
1: Veterinarian, Master’s in Pathology, Department of Pathology, Faculty of Veterinary Medicine and Animal Science of Botucatu, São Paulo State University (UNESP).
2: Veterinarian and Zootecnist, Department of Pathology, Faculty of Medicine of Botucatu, São Paulo State University (UNESP).Distrito de Rubião Jr., s/n – Cxa. Postal 560, Botucatu, São Paulo, CEP: 18618-000. Telephone: +55(14)997521341. Email: carlos.gonzalez-zambrano@unesp.br
3,4: Undergraduate student, Department of Pathology, Faculty of Veterinary Medicine and Animal Science of Botucatu, São Paulo State University (UNESP).
5: Veterinarian, Master’s and Ph.D. in Pathology, Department of Pathology, Faculty of Veterinary Medicine and Animal Science of Botucatu, São Paulo State University (UNESP). Email: noeme.rocha@unesp.br
*Distrito de Rubião Jr., s/n – Cxa. Postal 560, Botucatu, São Paulo, CEP: 18618-000. Telephone: +55(14)997521341. Email: carlos.gonzalez-zambrano@unesp.br
