OCORRÊNCIA DE MICRORGANISMOS ISOLADOS DE PERNA PERNA (LINNAEUS, 1758) DE UMA FAZENDA DE PRODUÇÃO EM TARITUBA, PARATY – RIO DE JANEIRO
REGISTRO DOI: 10.69849/revistaft/cl10202510111346
Antonia Lúcia dos Santos1
Vilma dos Santos2
Rafael Carracena de Souza Tapajóz3
Joana Angélica Barbosa Ferreira4
André Luis Almeida Souza5
Salvatore Giovanni de Simone6
Hilda do Nascimento Nobrega7
Abstract
Bivalve mollusks are invertebrates of great economic importance in several regions of the world. Given their commercial importance, this study aimed to monitor the hygiene and sanitary quality of Perna perna (Linnaeus, 1758), brown mussels, sourced from farms and harvests in Tarituba, Paraty, Rio de Janeiro. Samples were collected monthly from January to December 2011, resulting in 360 animals analyzed according to the microbiological parameters recommended by the 7th edition of the Brazilian Pharmacopoeia. Only 22.2% of the samples met the satisfactory quality criteria for consumption, according to the National Program for Safe Bivalve Mollusks (MoluBiS). The results reinforce the need to implement this program in the state of Rio de Janeiro to ensure the sanitary safety and quality of mollusks intended for human consumption.
Keywords: Bivalve mollusk. Microbiological. Contamination. Coliforms.
1. INTRODUCTION
Aquaculture has strengthened its central role in global food security, accounting for 46% of global fish production in 2018, corresponding to 82.1 million tons (FAO, 2015; 2020). Of this total, 17.7 million tons came from mollusks, with over 90% being bivalves. This group, in addition to its significant economic importance, is also ecologically crucial as natural filter feeders in marine ecosystems. In Santa Catarina, monitoring of malacoculture is progressing despite the lack of national legislation, thanks to a partnership between the government, producers, and universities (SOUZA & PETCOV, 2013).
Among the bivalves farmed in Brazil, the Perna perna (Linnaeus, 1758), the brown mussel, is widely distributed between Espírito Santo and Rio Grande do Sul. It is used as a bioindicator of environmental quality due to its ability to filter pollutants. However, this characteristic also favors the bioaccumulation of microorganisms and toxic substances, which can compromise food safety. However, the species has socioeconomic significance, supporting coastal populations economically, and is essential for local subsistence (BRASIL, 2020; RESGALLA Jr., WEBER & CONCEIÇÃO, 2008).
In addition to the species’ socioeconomic significance, bivalve consumption is growing due to its high nutritional value, coupled with the misperception of marine abundance (LEGAT et al., 2017). Between 1960 and 2018, global fish consumption doubled in proportion to population growth, reinforcing concerns about sustainability and the risk of depletion of marine resources (FAO, 2020).
From a One Health perspective, assessing microbiological contamination in bivalves plays a strategic role, as organisms such as Escherichia coli serve as indicators of fecal pollution and directly reflect anthropogenic impacts on coastal environments. These risks are exacerbated by inadequate management practices and poor water quality in cultivation areas, factors that increase the likelihood of pathogen transmission of health concern (ANON, 2017; FELDHUSEN, 2000; WALKER et al., 2018).
Given the growing demand for marine organisms, microbiological monitoring of bivalves and their cultivation areas is crucial to ensure food security and sustainable production (LEAL & FRANCO, 2008). Ilha Grande Bay, located in Tarituba (Paraty, RJ), was chosen because it features a complex ecosystem where preserved areas coexist with high-impact activities, such as oil terminals and nuclear power plants. Pathogens such as E. coli, Salmonella spp., Staphylococcus spp., and Vibrio spp. have already been detected in mollusks in the state, posing health risks (ANDRADE et al., 2022). Previous studies indicate the presence of Cryptosporidium spp. in Angra dos Reis and Vibrio parahaemolyticus in Niterói, even in farmed mussels. According to Brazilian legislation (BRASIL, 2012), live mollusks must be inspected before consumption. Mussels were chosen because they are widely used in environmental monitoring and are resistant to various conditions.
In this context, this study aimed to evaluate the microbiological quality of the P. perna mussel collected in Tarituba, focusing on the detection of bacteria indicative of fecal contamination. Biomonitoring of this species, widely used in environmental studies, aims to contribute to coastal management strategies, prevent foodborne diseases, and promote sustainable mariculture in areas impacted by human activities.
2. MATERIALS AND METHODS
2.1. Sampling – Sample Collection, Transportation, and ProcessingBetween January 2011 and December 2011, twelve monthly collections (n = 360) were conducted, with samples consisting of 30 P. perna mussels, collected from culture ropes (in natura) at the BEMAR farm, located in Tarituba, Paraty, Rio de Janeiro (Southeastern Brazil; 20°03’51” S, 44°03’45” W). The area is considered environmentally preserved, without anthropogenic interference (Figure 1).
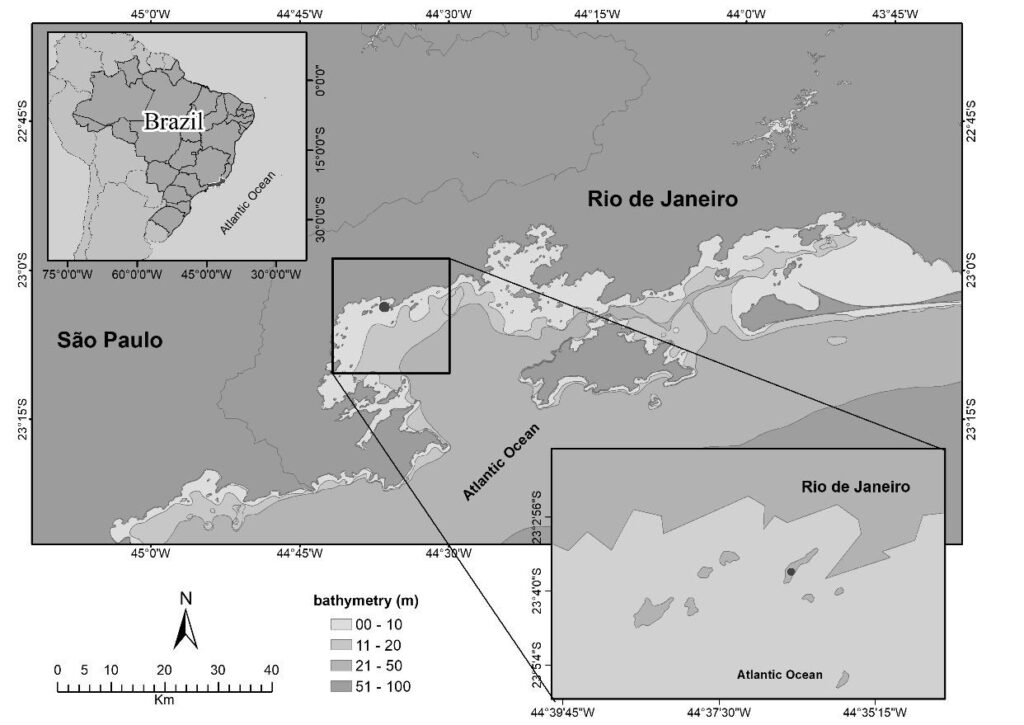
Figure 1: Tarituba is a district of Paraty, on the coast of Rio de Janeiro, located between the historic center of Paraty and Angra dos Reis, along the Rio Santos Highway (BR-101).
The mussels were collected manually, washed with seawater on site, and packaged with the valves closed in sterile polyethylene bags, labeled with the date and location. The samples were transported in thermal boxes (6-10°C) to the Oswaldo Cruz Foundation (FIOCRUZ) laboratories (National Institute for Quality Control in Health – INCQS and Center for Technological Development in Health – CDTS). At the laboratory, the animals underwent external washing and sanitization with a chlorine solution (5 ppm of CRL), in accordance with current health legislation (BRASIL, 2012), and were packaged in sterile containers until processing. Microbiological analyses were conducted in accordance with the INCQS/FIOCRUZ SOPs, as outlined in the Brazilian Pharmacopoeia – 7th ed. (BRASIL, 2024): verification of inhibitory capacity (POP 65.3210.009), pathogen screening (POP 65.3210.008), and enumeration of bile-tolerant bacteria (POP 65.3210.010). For the tests, the valves were opened with a sterile spatula, and tissues from the branchial arch, muscle, and intestine were dissected for bacteriological analysis.

Figure 2: Mussel rope.
Under aseptic conditions, the valves were opened with a sterile spatula, allowing the adductor muscle to be detached and the soft tissues to be accessed. The branchial arch tissue was removed with forceps and a scalpel.
3. RESULTS AND DISCUSSION
3.1. Isolation and Identification of Microorganisms
360 legs were collected from a farm in Tarituba, Paraty, Rio de Janeiro, and the gill arch, intestine, and muscle tissues were analyzed. The study evaluated the microbiological quality of mollusks, in a total of n = 545 species as analyzed, the most prevalent in Figure 3, Enterobacter aerogenes 44 / 8.1%, Enterobacter cloacae 29 / 5.3%, Enterobacter freundii 1 / 0.2%, Enterobacter sakazaii 6 / 1.1%, Citrobacter freundii 28 / 5.1%, Citrobacter aerogenes 1 / 0.2%, Citrobacter diversus 27 / 5.0%, Klebsiella planicola 2 / 0.7%, Klebsiella oxytoca 1 / 0.2%, Salmonella spp. 28 / 5.1%.
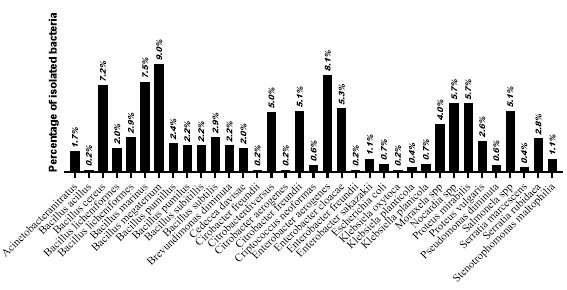
Figure 3: Prevalence of bacterial isolates in P. perna mussel tissues.
The results indicated a high level of contamination, with microbial counts exceeding the limits set by the Brazilian Pharmacopoeia – 7th ed. (BRASIL, 2024), even in the absence of specific pathogens. The data underscore the importance of microbiological control in ensuring food safety.The microbiological analysis revealed a high concentration of microorganisms in the intestinal tissue of P. perna, suggesting that this region acts as a reservoir for contaminants, possibly linked to poor sanitary conditions in the cultivation environment. The findings underscore the importance of monitoring the production chain and ensuring traceability to maintain food safety. We can see in Figure 4 that the principal genera identified were Citrobacter spp., Enterobacter spp., Proteus spp., Salmonella spp., and Bacillus spp., with emphasis on: C. diversus (8.9%), C. freundii (8.3%), and C. davisae (4.2%); E. aerogenes and E. cloacae (both 8.9%), and E. sakazakii (2.4%); P. mirabilis (8.9%) and P. vulgaris (7.7%); Salmonella spp. (8.9%); B. marinus and B. megaterium (7.7%), B. cereus (4.8%), and B. pumilus (4.2%). E. coli (2.4%), Nocardia spp. (2.4%), K. oxytoca (0.6%), and Serratia marcescens (0.6%) were also found.
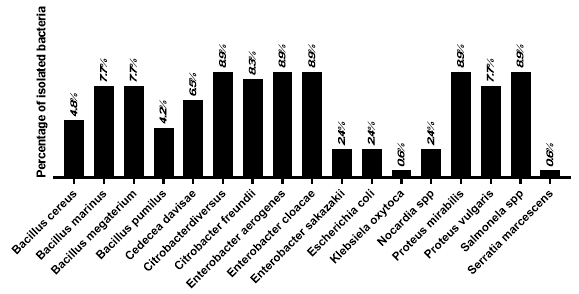
Figure 4: Prevalence of bacterial isolates in P. perna mussel intestinal tissue.
The microbiological analysis of the gill arch of P. perna revealed broad microbial diversity, highlighting species from the genera Bacillus, Citrobacter, Enterobacter, Serratia, Nocardia, Pseudomonas, and Salmonella. The highest prevalence was observed in Figure 5, with B. cereus (6.8%), C. freundii (6.4%), S. rubidaea (6.4%), Nocardia spp. (6.4%), and E. aerogenes (5.9%). Other relevant microorganisms included Salmonella spp. (5.5%), Moraxella spp. (5.5%), Brevundimonas diminuta (5.1%), and P. fluorescens (3.8%). These findings suggest that the gill arch, due to its role in filtration and respiration, is a critical point of exposure to environmental contaminants, serving as a sensitive indicator of microbiological water quality. The results underscore the importance of monitoring this structure in food biosafety and environmental assessment studies.
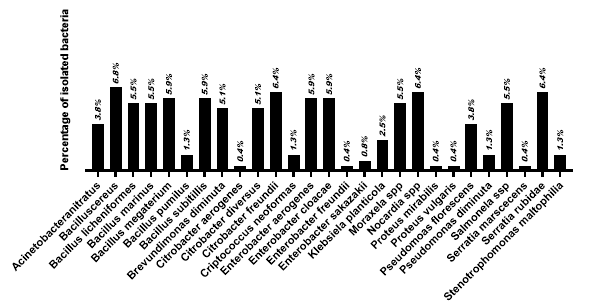
Figure 5: Prevalence of bacterial isolates in P. perna mussel gill arch tissue.
The microbiological analysis of P. perna muscle tissue revealed a significant presence of microorganisms, particularly E. aerogenes (10.6%), Proteus mirabilis (10.6%), and Nocardia spp. (9.2%). Species of the genus Bacillus, including B. megaterium, B. cereus, B. marinus, B. pumilus, B. subtilis, and B. licheniformis, were also identified, with prevalence rates ranging from 4.2% to 9.2%, in addition to Stenotrophomonas maltophilia (2.1%), as we can see in Figure 6. Although less exposed to the external environment, muscle tissue has also been shown to be a potential reservoir of microorganisms, posing a health risk, especially when consuming raw or undercooked shellfish. These results reinforce the importance of strict health controls throughout the production and commercial chain.
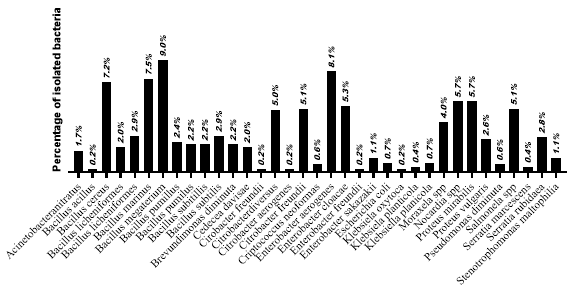
Figure 6: Prevalence of bacterial isolates in P. perna mussel muscle tissue.
This study of P. perna mussels allowed the isolation and identification of Gram-negative bacteria species (n=545), E. coli, K. oxytoca, C. freundii, P. mirabilis, P. vulgaris, Pseudomonas diminuta, Salmonella spp., among others, as shown in Figure 3.
The Tarituba region of Paraty, located near Ilha Grande Bay, features a small farm that produces Perna spp. mussels from Rio de Janeiro. Since 2007, Crop mortality has been reported in Angra dos Reis, possibly related to high water temperatures and weather events, although the exact cause remains unknown. In previous years, bacterial contamination was suspected as the culprit. Between 2009 and 2010, the municipality of Angra dos Reis experienced heavy rainfall that caused environmental disasters, including landslides, destruction of homes, and fatalities.
Between January and December 2011, sampling in Tarituba revealed a densely populated area with heavily infected mussels. The lack of basic sanitation in the region underscores the importance of these animals for public health, as they have a direct impact on environmental quality. Bivalve mollusks, being filter-feeding organisms, accumulate contaminants present in the water (HENRIQUES, 2004; JAY, 2000; MOLINS, 2001; NEWMAN et al., 2021; RODRIGUES, 1998; RODRIGUES-ARIZA et al., 1992). Several authors warn of the risks of consuming these mollusks, especially when raw or undercooked, due to contamination of the environment in which they live (BEIRÃO, TEIXEIRA & MEINERT, 2000; CARDONHA, CASIMIRO & VIEIRA, 1994; COOK, 1991; JOSÉ, 1996; ORDÓÑEZ et al., 2000; REICHENBACH-KLINKE & ELKAN, 1980; SANTOS et al., 2018).
In Ilha Grande Bay, sanitation is precarious; the area is heavily exploited for tourism, and some local beaches are densely populated. Detailed anatomical analysis by dissection, as described by Amaral (2010), is essential for informed management decisions during cultivation, with genomic differentiation being more accurate but also costlier. Aquatic microbiota adapts strongly to the physical and chemical conditions of the environment, with greater nutritional and thermal tolerance compared to their role in animal infections. Coliforms, for example, have low salinity resistance, and their presence in marine environments indicates recent and ongoing fecal contamination (VIEIRA et al., 2008).
Mussel collection sites, traditionally used by fishing communities, concentrate bivalves typical of estuarine and intertidal habitats, with mechanisms of tolerance to environmental stress (LI et al., 2017). Consumption of these contaminated organisms poses a public health risk due to the possible ingestion of pathogens or toxins (BALLESTEROS et al., 2018; BARROS et al., 2005; VIEIRA et al., 2008). By accumulating these elements, bivalve mollusks serve as environmental bioindicators, as their immune systems respond to changes detectable through analysis (FERREIRA et al., 2013; VIEIRA, 2003).
Government actions at the municipal, state, and federal levels-such as health education, public policies with subsidies, and training for fishermen-are essential to promote sustainable income and good hygiene practices, integrating public health, production, and environmental preservation within the One Health concept. However, despite domestic jets being released into water bodies and pollution resistance, public health advances in these communities remain limited.
The identification of Gram-negative bacterial species in the gills of bivalves collected in Tarituba, combined with the presence of domestic sewage in the area, highlights severe environmental contamination and associated health risks from consuming these organisms. Public policies focused on sustainable ecosystem management, aligned with the One Health concept, are crucial for protecting public health and ensuring the livelihoods of local communities.
4. CONCLUSIONS
Although the brown mussel, P. perna, is common along the coast of southern and southeastern Brazil, where it is widely cultivated, data are available on the association of various microorganisms, such as bacteria, with this mussel.
This study identified several bacteria in the P. perna samples analyzed, including the following taxa: E. coli, K. oxytoca, C. freundii, P. mirabilis, P. vulgaris, P. diminuta, and Salmonella spp.
These findings highlight potential public health risks and underscore the importance of systematic monitoring in the study area to ensure the safe harvesting and consumption of these bivalves.
Acknowledgments: We sincerely thank the Tarituba Institute team and its partnership with the Microorganisms Reference Laboratory of the INCQS/FIOCRUZ. We also thank Sergio Carvalho Moreira for producing Figure 1 and Vilma dos Santos for her significant assistance in the laboratory. The execution of this work was made possible thanks to the support of Coordination for the Improvement of Higher Education Personnel (CAPES) – Finance Code 001, with a scholarship for Rafael Carracena de Souza Tapajóz.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
REFERENCES
AMARAL, V. S. Estudo morfológico comparativo do gênero Crassostrea (Bivalvia: Ostreidea) do Atlântico oeste. 2010. 99 p. Dissertation (Master’s in Zoology) – Institute of Biosciences, University of São Paulo, São Paulo, 2010.
ANDRADE, N. M.; CARVALHO, A. M.; SALEH, A. M.; FONSECA, A. B. M.; MESQUITA, E. F. M.; ASNOWSI, M. C.; DUARTE, H.; CALIXTO, F. A. A.; NASCIMENTO, E. R. Hygiene conditions of mussels Perna perna captured in Niterói, RJ, Brazil: thermal intervention and microbiological evaluation. Food Sci. Technol., Campinas, v. 42, e107421, 2022.
ANON. Microbiological monitoring of bivalve mollusc harvesting areas. In: ANON. Guide to good practice: technical application. Issue 6. [S. l.]: [s. n.], 2017. Available at: http://www.eurlcefas.org
BALLESTEROS, E. R.; ANDRADE, V.; BARBIERI, E.; PINTO, A. B.; OLIVEIRA, R. S.; OLIVEIRA, A. J. F. C. Microbiological quality of oysters (Crassostrea sp) and water collected from farms and natural banks in Cananéia (SP). Bol. Inst. Pesca, v. 42, n. 1, p. 134-144, 2018.
BARROS, L. M. O.; THEOPHILO, G. N. D.; COSTA, R. G.; RODRIGUES, D. P.; VIEIRA, R. H. S. F. Fecal contaminant of the oyster Crassostrea rhizophorae commercialized at Praia do Futuro, Fortaleza, Ceará State. Ver. Cienc. Agron., v. 36, n. 3, p. 285-289, 2005.
BEIRÃO, H.; TEIXEIRA, E.; MEINERT, E. M. Processamento e industrialização de moluscos. In: SEMINÁRIO E WORKSHOP TECNOLOGIAS PARA APROVEITAMENTO INTEGRAL DO PESCADO, 1., 2000, Campinas. Anais… Campinas: ITAL, 2000. p. 38-84.
BRASIL. Agência Nacional de Vigilância Sanitária. Farmacopéia Brasileira. 7ª ed. Brasília: ANVISA, 2025.
_____. Ministério da Agricultura, Pecuária e Abastecimento. Boletim de resultados do acompanhamento dos contratos de cessão de uso da maricultura em águas da União. Boletim da Maricultura em Águas da União 2017-2018-2019. Portaria SAP/MAPA. Brasília, DF: Secretaria de Aquicultura e Pesca, 2020.
_____. Ministério da Pesca e Aquicultura. Institui o Programa Nacional de Controle Higiênico-Sanitário de Moluscos Bivalves (PNCMB), estabelece os procedimentos para a sua execução e dá outras providências. Instrução Normativa Interministerial nº 7, de 8 de maio de 2012. Diário Oficial [da] República Federativa do Brasil, Brasília, DF, 9 maio 2012.
CARDONHA, A. M. S.; CASIMIRO, A. R. S.; VIEIRA, R. H. S. F. Identificação de bactérias psicotróficas em caudas de lagosta, durante processo industrial com tripolifosfato de sódio. Higiene Alimentar, São Paulo: Impress, v. 8, n. 31, p. 29-34, 1994.
COOK, D. W. Microbiology of bivalve molluscan shellfish. In: WARD, D. R.; HACKNEY, C. Microbiology of marine food products. New York: Van Nostrand Reinhold, 1991. cap. 2, p. 19-34.
FAO. Brazil – National Aquaculture Sector Overview. Rome: Food and Agriculture Organization, 2015. Available at: http://www.fao.org/fishery/countrysector/naso_brazil/en
_____. The State of World Fisheries and Aquaculture 2020 (SOFIA): Sustainability in action. Rome: Food and Agriculture Organization, 2020.
FELDHUSEN, F. The role of seafood in bacterial foodborne diseases. Microbes and Infect., v. 2, p. 1651-1660, 2000.
FERREIRA, M. S.; MÁRSICO, E. T.; CONTE JUNIOR, C. A.; MARQUES JÚNIOR, A. N.; MANO, S. B.; SÃO CLEMENTE, S. C. Trace metal contamination in mussel Perna perna from Brazilian coast. Cienc. Rural, Niterói, v. 43, n. 6, p. 1012-1010, 2013.
HENRIQUES, M. B. Resistência do mexilhão Perna perna (Linnaeus, 1758) proveniente de bancos naturais da baixada santista, a variações de temperatura, salinidade, tempo de exposição ao ar e determinação da incidência de parasitismo. 2004. 102 p. Thesis (Doctorate in Biological Sciences) – Institute of Biosciences, São Paulo State University “Júlio de Mesquita Filho”, Rio Claro, 2004.
JAY, J. M. Modern Food Microbiology. 6th ed. Gaithersburg, Maryland: Aspen Publishers, 2000. 679 p.
JOSÉ, V. F. Bivalves e a segurança do consumidor. 1996. 157 p. Dissertation (Master’s in Environmental Science) – University of São Paulo, São Paulo, 1996.
LEAL, D. A. G; FRANCO, M. Bivalve molluscs destined for human consumption as vectors of pathogenic protozoa: Detection methodologies and control rules. Ver. Panam. Infectol., v. 10, p. 48-57, 2008.
LEGAT, J. F. A.; PUCHNICK-LEGAT, A.; FOGAÇA, F. H. S.; TURECK, C. R.; SUHNEL, S.; MELO, C. M. R. Growth and survival of bottom oyster Crassostrea gasar cultured in the Northeast and South of Brazil. B. Inst. Pesca, v. 43, n. 2, p. 172-184, 2017.
LI, J.; ZHANG, Y.; MAO, F.; TONG, Y.; LIU, Y.; ZHANG, Y.; YU, Z. Characterization and identification of differentially expressed genes involved in thermal adaptation of the Hong Kong oyster Crassostrea hongkongensis by digital gene expression profiling. Front. Mar. Sci., v. 4, n. 112, p. 1-12, 2017.
NEWMAN, L.; BARBOSA, A. C.; LEITE, A. M. O.; SOUZA, M. D. P.; MOLISANI, M. M. Chemical and microbiological characterization of bivalve mollusks collected, cultivated and marketed at the State of Rio de Janeiro coast. Arq. Cien. Mar, Fortaleza, v. 54, n. 1, p. 122-134, 2021.
MOLINS, R. A. Food Irradiation: Principles and Applications. New York: John Wiley & Sons, 2001. 496 p.
ORDÓÑEZ, J. A.; RODRÍGUEZ, M. I. C.; ÁLVAREZ, L. F.; SANZ, M. L. G.; MINGUILLÓN, G. D. G. F.; PERALES, L. H.; CORTECERO, M. D. S. Tecnología de los alimentos: alimentos de origen animal. Madrid: Ediciones Síntesis, 2000. v. 2. 294 p.
PEREIRA, C. S.; POSSAS, C. D. A.; VIANA, C. M.; RODRIGUES, D. D. P. Characteristics of Vibrio parahaemolyticus isolated from mussels (Perna perna) commercialized at Niterói, Rio de Janeiro. Ver. Soc. Bras. Med. Trop., v. 40, n. 1, p. 56-59, 2007.
REICHENBACH-KLINKE, H. H.; ELKAN, E. Diseases of Fish. Oxford: Butterworth-Heinemann, 1980. 482 p.
RESGALLA Jr., C.; WEBER, L. I.; CONCEIÇÃO, M. B. O mexilhão Perna perna (L.): biologia, ecologia e aplicações. Rio de Janeiro: Interciência, 2008. 323 p.
RODRIGUES, P. F. Caracterização sanitária de áreas de criação de moluscos bivalvos do litoral norte do Estado de São Paulo. 1998. 66 p. Dissertation (Master of Science) – University of São Paulo, São Paulo, 1998.
RODRIGUES-ARIZA, A.; ABRIL, N.; NAVAS, J. I.; DORADO, G.; LÓPEZ-BAREA, J.; PUEYO, C. Metal, mutagenicity, and biochemical studies on bivalve mollusks from the Spanish coasts. Environ. Mol. Mutagen., New York: Wiley-Liss, v. 19, p. 112-124, 1992.
SANTOS, A. L.; OLIVEIRA, L. T. F. de; SOUZA, A. L. A.; HAUSER DAVIS, R.; SIMONE, S. G. de. Cryptosporidium spp. contamination in Perna perna mussels destined for human consumption in Southeastern Rio de Janeiro, Brazil. Bull. Environ. Contam. Toxicol., n. 100, p. 240-244, 2018.
SOUZA, R. V. de; PETCOV, H. F. D. Comércio legal de moluscos bivalves. Florianópolis: Epagri, 2013. 58 p. (Epagri. Boletim Didático, n. 95).
VIEIRA, R. H. S. F.; ATAYDE, M. A.; CARVALHO, E. M. R.; CARVALHO, F. C. T.; FONTELES FILHO, A. A. Contaminação fecal da ostra Crassostrea rhizophorae e da água de cultivo do estuário do Rio Pacoti (Eusébio, Estado do Ceará): isolamento e identificação de Escherichia coli e sua susceptibilidade a diferentes antimicrobianos. Braz. J. Vet. Res. Anim. Sci., v. 45, n. 3, p. 180-189, 2008.
VIEIRA, R. H. S. F. Microbiologia, higiene e qualidade do pescado: teoria e prática. São Paulo: Varela, 2003.
WALKER, D. L.; YOUNGER, A. L.; STOCKL, L.; EYBAER-AUSTIN, C. Escherichia coli testing and enumeration in live bivalve shellfish – present methods and future directions. Food Microbiol., v. 73, p. 29-38, 2018.
1MSc., Oswaldo Cruz Foundation (FIOCRUZ), Center for Technological Development in Health (CDTS). Brasil Avenue, 4365 – Manguinhos Campus, Manguinhos – Rio de Janeiro/RJ, Brazil. Zip Code: 21040-900 – santoslucia1303@gmail.com
2BSc., Laboratory of Epidemiology and Molecular Systematics (LESM), Oswaldo Cruz Institute (IOC), Oswaldo Cruz Foundation (FIOCRUZ). Brasil Avenue, 4365 – Manguinhos Campus, Manguinhos – Rio de Janeiro/RJ, Brazil. Zip Code: 21040-900 – vssantos.vilma@gmail.com
3MSc., Oswaldo Cruz Foundation (FIOCRUZ), Center for Technological Development in Health (CDTS). Brasil Avenue, 4365 – Manguinhos Campus, Manguinhos – Rio de Janeiro/RJ, Brazil. Zip Code: 21040-900 / Graduate Program in Science and Biotechnology (PPBI), Institute of Biology (IB), Fluminense Federal University (UFF). Prof. Marcos Waldemar de Freitas Reis Street, Gragoatá Campus, São Domingos – Niterói/RJ, Brazil. Zip Code: 24210-201 – rafaeltapajoz@gmail.com
4Dra., Non-Sterile Products Sector, Department of Microbiology, National Institute for Quality Control in Health (INCQS), Oswaldo Cruz Foundation (FIOCRUZ). Brasil Avenue, 4365 – Manguinhos Campus, Manguinhos – Rio de Janeiro/RJ, Brazil. Zip Code: 21040-900 – joana.barbosa@fiocruz.br
5Dr., Multidisciplinary Laboratory of Biochemistry Teaching (LMEB), Iguaçu University (UNIG). Abílio Augusto Távora Avenue, 2134, Luz – Nova Iguaçu/RJ, Brazil. Zip Code: 26260-045 – drandleish@gmail.com
6Dr., Oswaldo Cruz Foundation (FIOCRUZ), Center for Technological Development in Health (CDTS). Brasil Avenue, 4365 – Manguinhos Campus, Manguinhos – Rio de Janeiro/RJ, Brazil. Zip Code: 21040-900 / Graduate Program in Science and Biotechnology (PPBI), Institute of Biology (IB), Fluminense Federal University (UFF). Prof. Marcos Waldemar de Freitas Reis Street, Gragoatá Campus, São Domingos – Niterói/RJ, Brazil. Zip Code: 24210-201 – salvatore.simone@fiocruz.br
7MSc., Non-Sterile Products Sector, Department of Microbiology, National Institute for Quality Control in Health (INCQS), Oswaldo Cruz Foundation (FIOCRUZ). Brasil Avenue, 4365 – Manguinhos Campus, Manguinhos – Rio de Janeiro/RJ, Brazil. Zip Code: 21040-900 – hilda.nobrega@fiocruz.br
